| Citation: | Zhu HT, Luo JX, Dai Q, Zhu SG, Yang H et al. Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group. Opto-Electron Adv 6, 230018 (2023). doi: 10.29026/oea.2023.230018 |
Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group
-
Abstract
Systemic blood circulation is one of life activity’s most important physiological functions. Continuous noninvasive hemodynamic monitoring is essential for the management of cardiovascular status. However, it is difficult to achieve systemic hemodynamic monitoring with the daily use of current devices due to the lack of multichannel and time-synchronized operation capability over the whole body. Here, we utilize a soft microfiber Bragg grating group to monitor spatiotemporal hemodynamics by taking advantage of the high sensitivity, electromagnetic immunity, and great temporal synchronization between multiple remote sensor nodes. A continuous systemic hemodynamic measurement technique is developed using all-mechanical physiological signals, such as ballistocardiogram signals and pulse waves, to illustrate the actual mechanical process of blood circulation. Multiple hemodynamic parameters, such as systemic pulse transit time, heart rate, blood pressure, and peripheral resistance, are monitored using skin-like microfiber Bragg grating patches conformally attached at different body locations. Relying on the soft microfiber Bragg grating group, the spatiotemporal hemodynamic monitoring technique opens up new possibilities in clinical medical diagnosis and daily health management. -
-
References
[1] Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet 395, 785–794 (2020). doi: 10.1016/S0140-6736(19)32007-0 [2] Kaur RI. Electrocardiogram signal analysis - an overview. Int J Comput Appl 84, 22–25 (2013). doi: 10.5120/14590-2826 [3] Debbal SM, Bereksi-Reguig F. Computerized heart sounds analysis. Comput Biol Med 38, 263–280 (2008). doi: 10.1016/j.compbiomed.2007.09.006 [4] Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rep 8, 14–25 (2012). doi: 10.2174/157340312801215782 [5] Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 28, R1–R39 (2007). doi: 10.1088/0967-3334/28/3/R01 [6] Buxi D, Redouté JM, Yuce MR. A survey on signals and systems in ambulatory blood pressure monitoring using pulse transit time. Physiol Meas 36, R1–R26 (2015). doi: 10.1088/0967-3334/36/3/R1 [7] Chen Y, Wen CY, Tao GC, Bi M. Continuous and noninvasive measurement of systolic and diastolic blood pressure by one mathematical model with the same model parameters and two separate pulse wave velocities. Ann Biomed Eng 40, 871–882 (2012). doi: 10.1007/s10439-011-0467-2 [8] Chen Y, Wen CY, Tao GC, Bi M, Li GQ. Continuous and noninvasive blood pressure measurement: a novel modeling methodology of the relationship between blood pressure and pulse wave velocity. Ann Biomed Eng 37, 2222–2233 (2009). doi: 10.1007/s10439-009-9759-1 [9] Ding XR, Yan BP, Zhang YT, Liu J, Zhao N et al. Pulse transit time based continuous cuffless blood pressure estimation: a new extension and a comprehensive evaluation. Sci Rep 7, 11554 (2017). doi: 10.1038/s41598-017-11507-3 [10] Mukkamala R, Hahn JO, Inan OT, Mestha LK, Kim CS et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng 62, 1879–1901 (2015). doi: 10.1109/TBME.2015.2441951 [11] Sharma M, Barbosa K, Ho V, Griggs D, Ghirmai T et al. Cuff-less and continuous blood pressure monitoring: a methodological review. Technologies 5, 21 (2017). doi: 10.3390/technologies5020021 [12] Koo JH, Yun HW, Lee WC, Sunwoo SH, Shim HJ et al. Recent advances in soft electronic materials for intrinsically stretchable optoelectronic systems. Opto-Electron Adv 5, 210131 (2022). doi: 10.29026/oea.2022.210131 [13] Bennett A, Beiderman Y, Agdarov S, Beiderman Y, Hendel R et al. Monitoring of vital bio-signs by analysis of speckle patterns in a fabric-integrated multimode optical fiber sensor. Opt Express 28, 20830–20844 (2020). doi: 10.1364/OE.384423 [14] Chung HU, Kim BH, Lee JY, Lee J, Xie ZQ et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019). doi: 10.1126/science.aau0780 [15] Chung HU, Rwei AY, Hourlier-Fargette A, Xu S, Lee K et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat Med 26, 418–429 (2020). doi: 10.1038/s41591-020-0792-9 [16] Jin Y, Chen GN, Lao KT, Li SH, Lu Y et al. Identifying human body states by using a flexible integrated sensor. npj Flex Electron 4, 28 (2020). doi: 10.1038/s41528-020-00090-9 [17] Li HC, Ma YJ, Liang ZW, Wang ZH, Cao Y et al. Wearable skin-like optoelectronic systems with suppression of motion artifacts for cuff-less continuous blood pressure monitor. Natl Sci Rev 7, 849–862 (2020). doi: 10.1093/nsr/nwaa022 [18] Zhong F, Hu W, Zhu PN, Wang H, Ma C et al. Piezoresistive design for electronic skin: from fundamental to emerging applications. Opto-Electron Adv (2022). doi: 10.29026/oea.2022.210029 [19] Li JH, Chen JH, Xu F. Sensitive and wearable optical microfiber sensor for human health monitoring. Adv Mater Technol 3, 1800296 (2018). doi: 10.1002/admt.201800296 [20] Wang CH, Li XS, Hu HJ, Zhang L, Huang ZL et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat Biomed Eng 2, 687–695 (2018). doi: 10.1038/s41551-018-0287-x [21] Zhang L, Pan J, Zhang Z, Wu H, Yao N et al. Ultrasensitive skin-like wearable optical sensors based on glass micro/nanofibers. Opto-Electron Adv 3, 190022 (2020). doi: 10.29026/oea.2020.190022 [22] Zhu HT, Zhan LW, Dai Q, Xu B, Chen Y et al. Self‐assembled wavy optical microfiber for stretchable wearable sensor. Adv Opt Mater 9, 2002206 (2021). doi: 10.1002/adom.202002206 [23] Hill KO, Meltz G. Fiber Bragg grating technology fundamentals and overview. J Lightwave Technol 15, 1263–1276 (1997). doi: 10.1109/50.618320 [24] Dziuda Ł, Skibniewski FW. A new approach to ballistocardiographic measurements using fibre Bragg grating-based sensors. Biocybern Biomed Eng 34, 101–116 (2014). doi: 10.1016/j.bbe.2014.02.001 [25] Haseda Y, Bonefacino J, Tam HY, Chino S, Koyama S et al. Measurement of pulse wave signals and blood pressure by a plastic optical fiber FBG sensor. Sensors 19, 5088 (2019). doi: 10.3390/s19235088 [26] Xu L, Liu N, Ge J, Wang XQ, Fok MP. Stretchable fiber-Bragg-grating-based sensor. Opt Lett 43, 2503–2506 (2018). doi: 10.1364/OL.43.002503 [27] Al-Fakih EA, Abu Osman NA, Mahamd Adikan FR, Eshraghi A, Jahanshahi P. Development and validation of fiber Bragg grating sensing pad for interface pressure measurements within prosthetic sockets. IEEE Sensors J 16, 965–974 (2016). doi: 10.1109/JSEN.2015.2495323 [28] Li TL, Su YF, Chen FY, Liao XQ, Wu Q et al. A skin‐like and highly stretchable optical fiber sensor with the hybrid coding of wavelength–light intensity. Adv Intell Syst 4, 2100193 (2022). doi: 10.1002/aisy.202100193 [29] Pan J, Zhang Z, Jiang CP, Zhang L, Tong LM. A multifunctional skin-like wearable optical sensor based on an optical micro-/nanofibre. Nanoscale 12, 17538–17544 (2020). doi: 10.1039/D0NR03446K [30] Ma SQ, Wang XY, Li P, Yao N, Xiao JL et al. Optical Micro/Nano fibers enabled smart textiles for human–machine interface. Adv Fiber Mater 4, 1108–1117 (2022). doi: 10.1007/s42765-022-00163-6 [31] Brambilla G, Finazzi V, Richardson DJ. Ultra-low-loss optical fiber nanotapers. Opt Express 12, 2258–2263 (2004). doi: 10.1364/OPEX.12.002258 [32] Lou JY, Wang YP, Tong LM. Microfiber optical sensors: a review. Sensors 14, 5823–5844 (2014). doi: 10.3390/s140405823 [33] Thomas J, Voigtländer C, Becker RG, Richter D, Tünnermann A et al. Femtosecond pulse written fiber gratings: a new avenue to integrated fiber technology. Laser Photonics Rev 6, 709–723 (2012). doi: 10.1002/lpor.201100033 [34] Luo JX, Liu S, Chen PJ, Lu SZ, Zhang Q et al. Fiber optic hydrogen sensor based on a Fabry-Perot interferometer with a fiber Bragg grating and a nanofilm. Lab Chip 21, 1752–1758 (2021). doi: 10.1039/D1LC00012H [35] Brambilla G, Xu F, Horak P, Jung Y, Koizumi F et al. Optical fiber nanowires and microwires: fabrication and applications. Adv Opt Photonics 1, 107–161 (2009). doi: 10.1364/AOP.1.000107 [36] Tong LM, Zi F, Guo X, Lou JY. Optical microfibers and nanofibers: a tutorial. Opt Commun 285, 4641–4647 (2012). doi: 10.1016/j.optcom.2012.07.068 [37] Yang LY, Li YP, Fang F, Li LY, Yan ZJ et al. Highly sensitive and miniature microfiber-based ultrasound sensor for photoacoustic tomography. Opto-Electron Adv 5, 200076 (2022). doi: 10.29026/oea.2022.200076 [38] Mukkamala R, Xu D. Continuous and less invasive central hemodynamic monitoring by blood pressure waveform analysis. Am J Physiol Heart Circ Physiol 299, H584–H599 (2010). doi: 10.1152/ajpheart.00303.2010 [39] Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37, 1236–1241 (2001). doi: 10.1161/01.HYP.37.5.1236 [40] Kim J, Song TJ, Song D, Lee KJ, Kim EH et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension 64, 240–246 (2014). doi: 10.1161/HYPERTENSIONAHA.114.03304 [41] Zhang GQ, Gao MW, Xu D, Olivier NB, Mukkamala R. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. J Appl Physiol 111, 1681–1686 (2011). doi: 10.1152/japplphysiol.00980.2011 [42] Rueckert PA, Slane PR, Lillis DL, Hanson P. Hemodynamic patterns and duration of post-dynamic exercise hypotension in hypertensive humans. Med Sci Sports Exerc 28, 24–32 (1996). doi: 10.1097/00005768-199601000-00010 [43] Ohlsson Å, Steinhaus D, Kjellström B, Ryden L, Bennett T. Central hemodynamic responses during serial exercise tests in heart failure patients using implantable hemodynamic monitors. Eur J Heart Fail 5, 253–259 (2003). doi: 10.1016/S1388-9842(02)00250-7 [44] Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 36, 312–318 (2000). doi: 10.1161/01.HYP.36.3.312 [45] Taylor AJ, Bobik A, Berndt MC, Ramsay D, Jennings G. Experimental rupture of atherosclerotic lesions increases distal vascular resistance: a limiting factor to the success of infarct angioplasty. Arterioscler Thromb Vasc Biol 22, 153–160 (2002). doi: 10.1161/hq0102.101128 -
Supplementary Information
Supplementary information for Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group 
-
Access History
Article Metrics
-
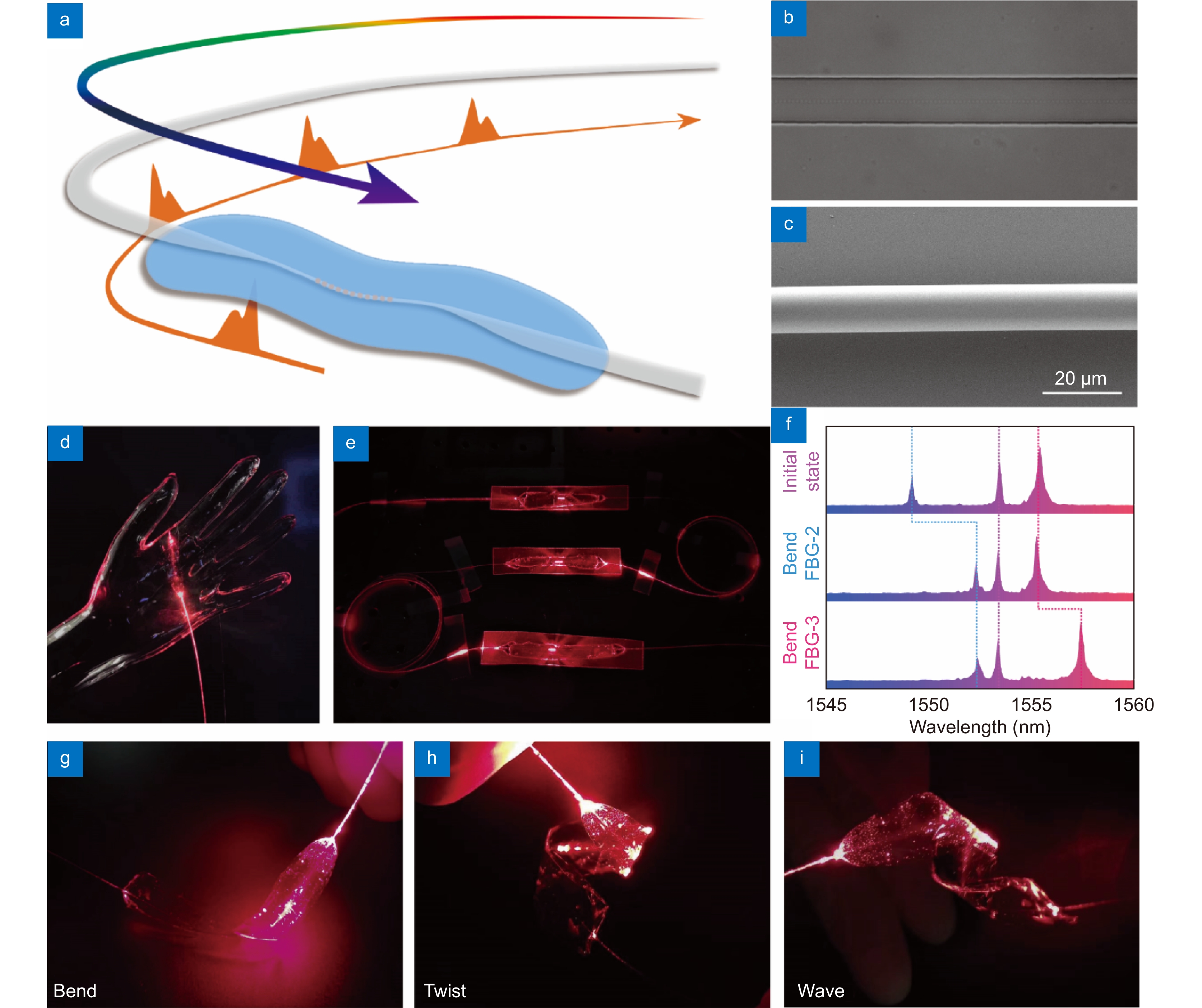
Figure 1.
Skin-like microfiber Bragg grating (μFBG) patch. (a) Schematic illustration of the soft μFBG patch. The optical microfiber contains a femtosecond laser-inscribed FBG embedded in a thin PDMS patch and is connected end-to-end with two commercial single-mode optical fibers (SMFs) for light propagation. (b) Optical micrograph of the soft μFBG patch. The FBG is inscribed in the axis of the microfiber. (c) SEM of the microfiber, which has a diameter of 12 μm. (d) Image of the soft μFBG patch draped on a life-sized, transparent mannequin hand conformally. (e) Image of three soft μFBG patches connected in series to illustrate the multichannel sensing capabilities. (f) Reflective optical spectrum of the three soft μFBG patches. The μFBG patches are capable of detecting strain separately without interference due to the different working wavelengths. (g–i) Images of the soft μFBG patch under modes of bending, twisting, and waving, demonstrating the mechanical compliance and robustness of the device.
-
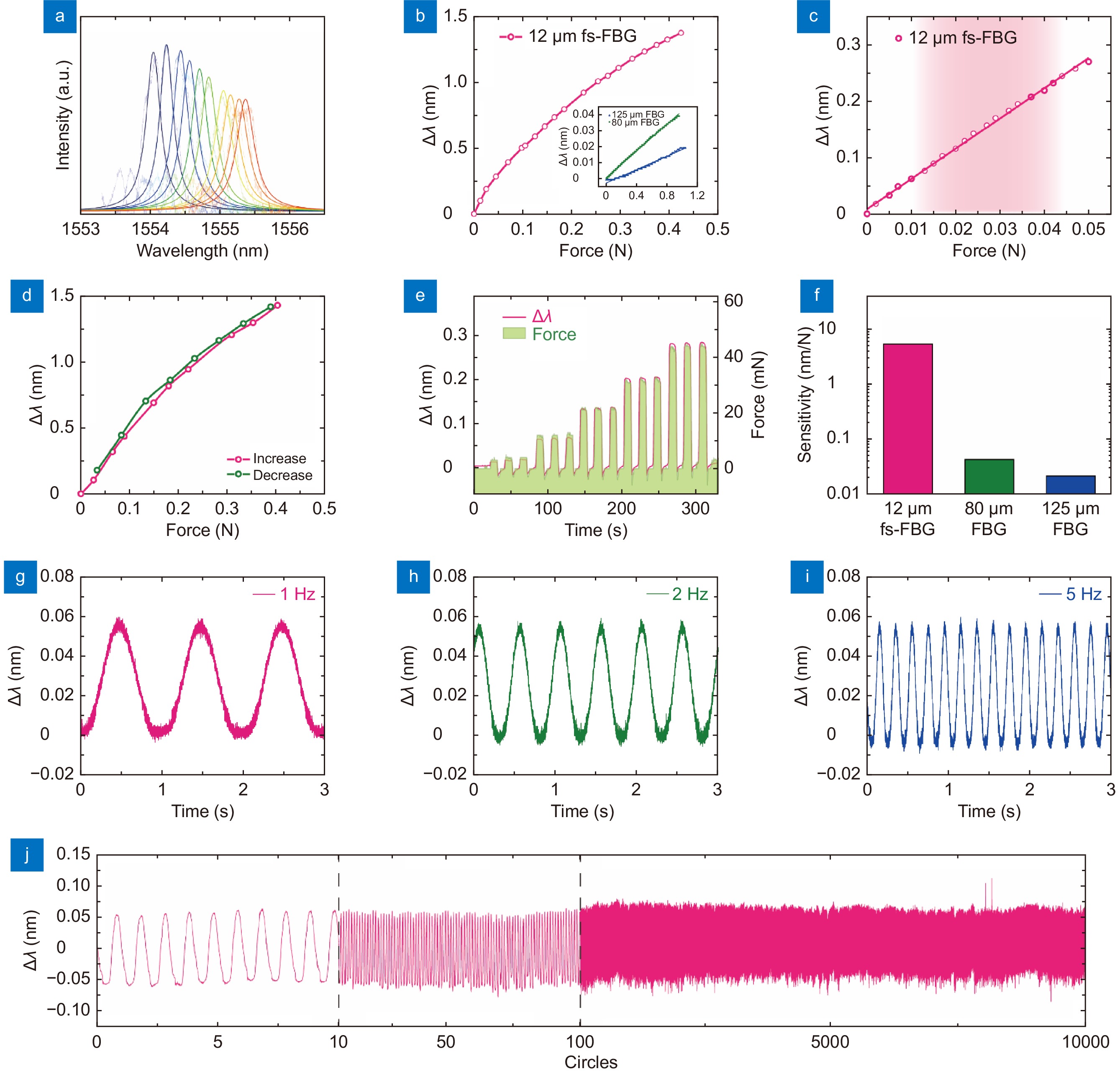
Figure 2.
Stress and vibration characterization of skin-like μFBG patch. (a) Reflective optical spectra of the soft μFBG patch under different stresses. (b) Stress response of the soft μFBG patch. (Inset: stress response of the commercial FBG with a diameter of 80 μm and 125 μm separately for comparison.) (c) Details of the stress response of the soft μFBG patch when detecting small stresses. The red region indicates the stress range of the life pulse. (d) Stress response of the soft μFBG patch when stress increases and decreases to illustrate the hysteresis property. (e) Dynamic stress response of the soft μFBG patch compared with a commercial force meter. (f) Sensitivity of the soft μFBG patch, which is enhanced by two orders of magnitude compared with commercial FBGs with diameters of 80 μm and 125 μm. (g–i) Vibration characterization of the soft μFBG patch under frequencies of 1 Hz, 2 Hz, and 5 Hz. (j) 10000-circle repetition tests of the soft μFBG patch to illustrate great repeatability.
-
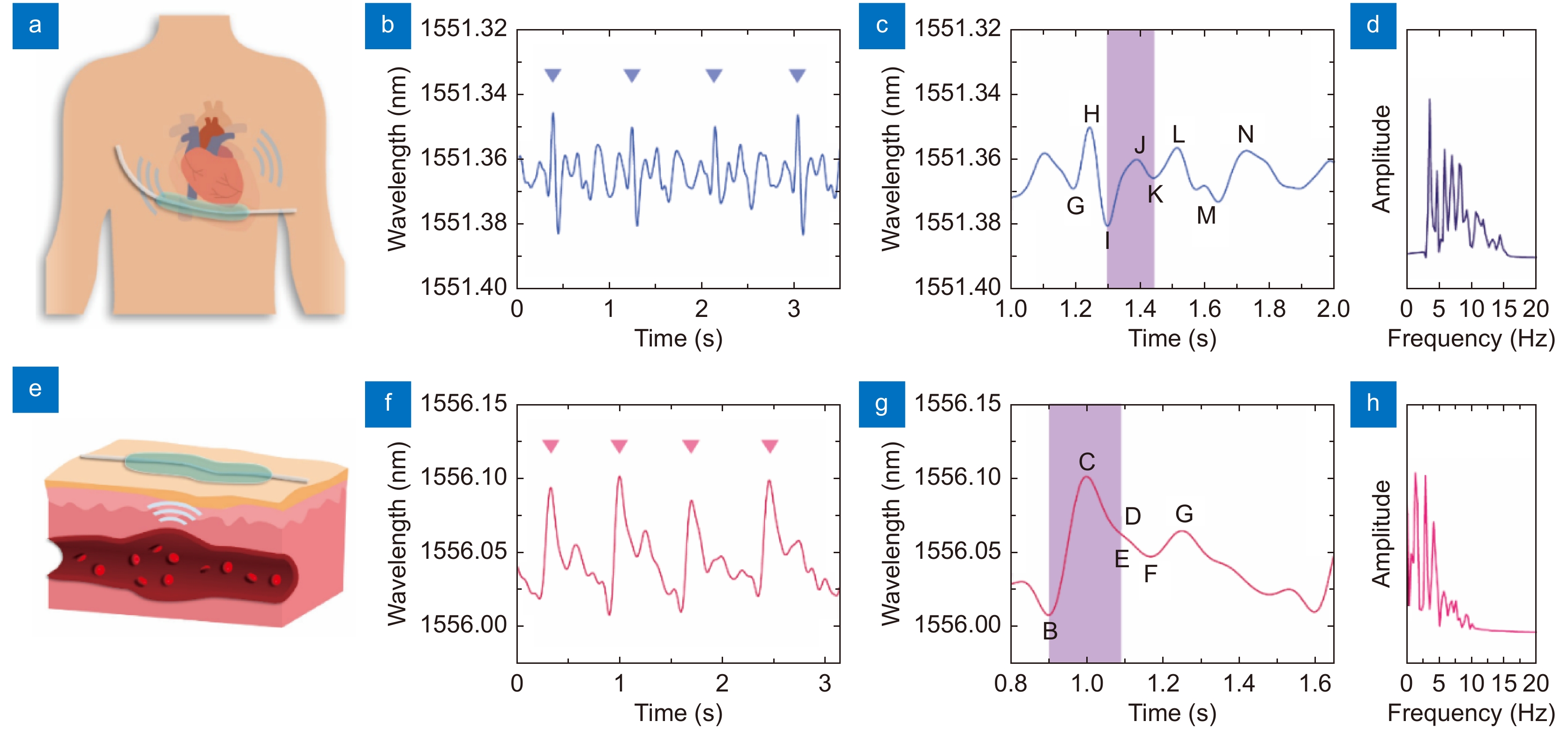
Figure 3.
Ballistocardiogram (BCG) signal and pulse wave detection using a μFBG patch. (a) Schematic diagram of BCG signal detection. A soft μFBG patch is attached to the chest skin at the tricuspid site. (b) BCG signal detected by the soft μFBG patch, and the triangle marks indicate the cardiac cycle. (c) Details of the BCG signal. The purple region indicates the systole of the rhythm of the heart, which can be identified according to the characteristic I wave and K wave. (d) Frequency spectrum of the BCG signal. (e) Schematic diagram of pulse wave detection. A soft μFBG patch is attached to the skin at the sites of the superficial arteries. (f) Pulse wave detected by the soft μFBG patch, and the triangle marks indicate the cardiac cycle. (g) Details of the pulse wave. The purple region indicates the systole of the rhythm of the heart, which can be identified according to the characteristic B wave and E wave. (h) Frequency spectrum of pulse wave.
-
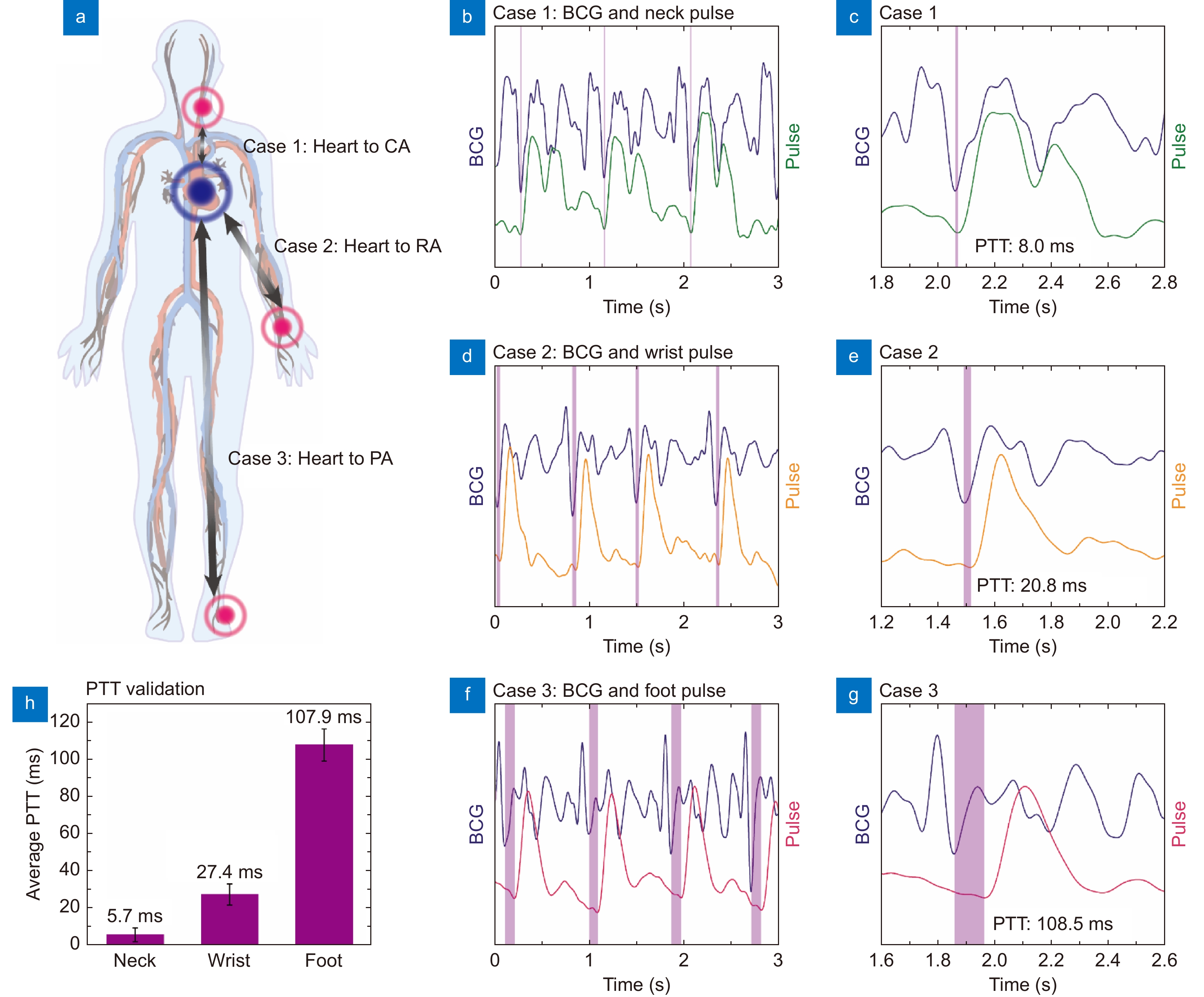
Figure 4.
Systemic pulse transmit time (PTT) calculation using the soft μFBG group. (a) Schematic diagram of the measurement positions for the three cases. Systemic PTTs are calculated via the cooperative analysis of BCG signals and pulse waves at the three different superficial artery sites. (b, c) Case 1: simultaneous measurement of BCG and pulse wave at the carotid artery (CA). (d, e) Case 2: simultaneous measurement of BCG and pulse wave at the radial artery (RA). (f, g) Case 3: simultaneous measurement of BCG and pulse wave at the pedal artery (PA). The graphs (c, e, g) show the details of the BCG signal and pulse wave within one cardiac cycle. The PTTs of the three cases, indicated by the purple region, are identified by the I wave of the BCG and the B wave of the pulse wave. (h) Comparison of the average PTT results of the three cases to illustrate the systemic hemodynamic evaluation ability of the soft μFBG group.
-
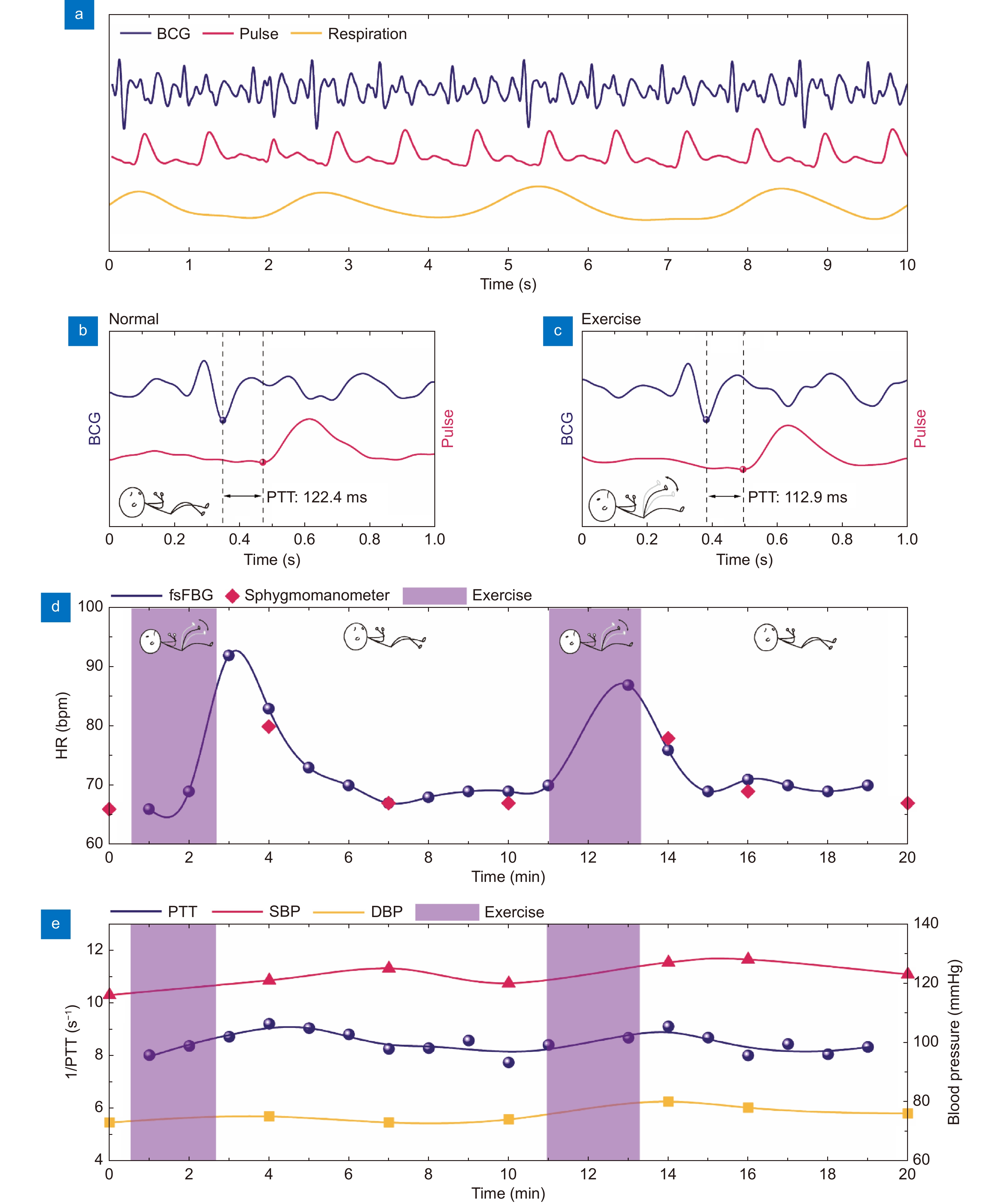
Figure 5.
Hemodynamic monitoring during exercise using soft μFBG group. (a) Representative BCG signal (blue), pulse wave at the PA site (red), and respiration signal (yellow) monitored simultaneously. (b and c) Details of the synchronized BCG signal and pulse wave during the normal state and exercise state, respectively. PTT is indicated by the dashed lines. (d) Comparison of heart rate (HR) calculated from the vital signal acquired by the μFBG group (blue) and a commercial sphygmomanometer (red). (e) Comparison of PTT (blue), which is calculated from the BCG signal and pulse wave acquired by the μFBG group, and the systolic and diastolic blood pressure (SBP and DBP) acquired from a commercial sphygmomanometer. The exercise periods are indicated by the purple region.
-
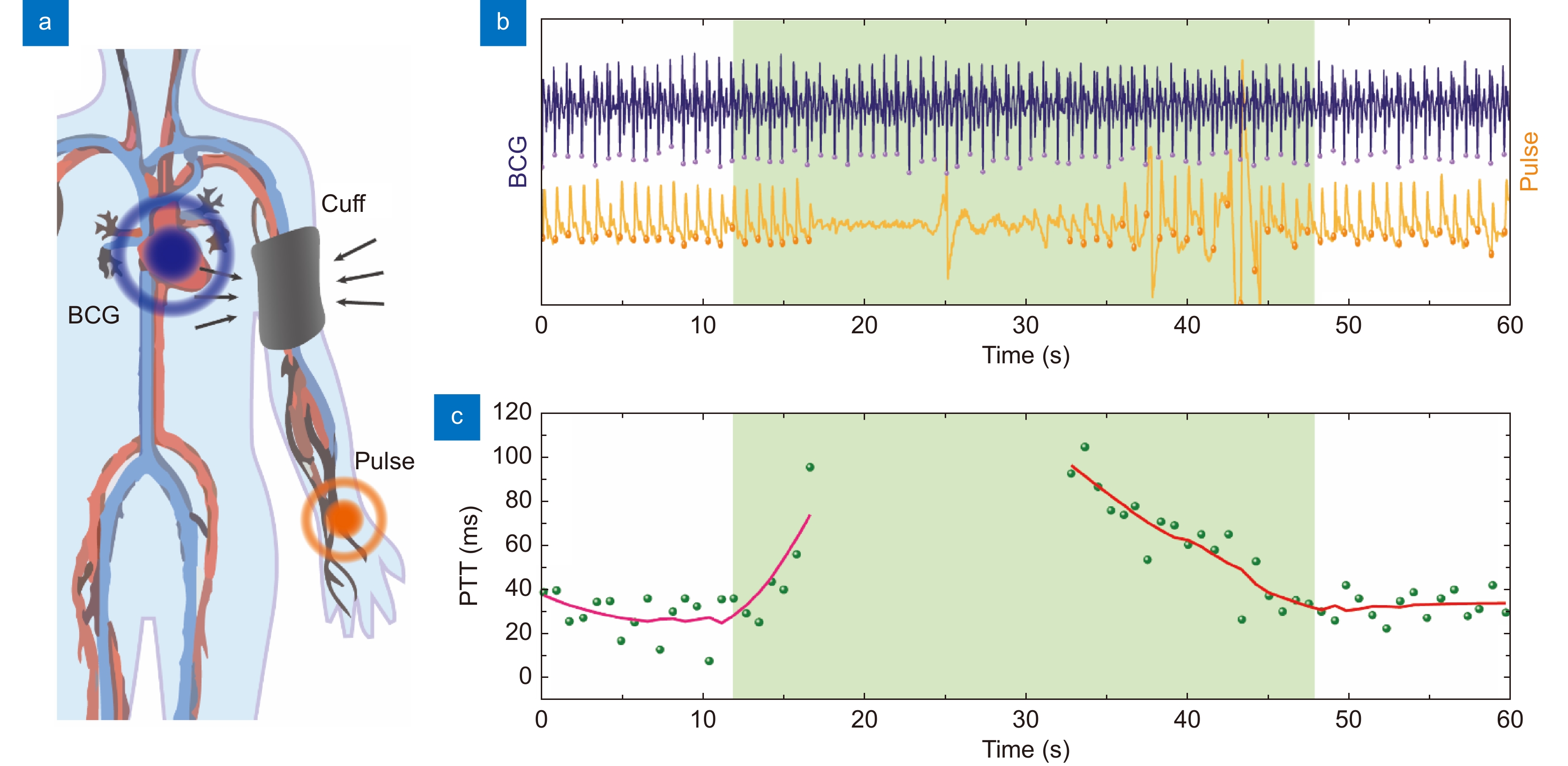
Figure 6.
Peripheral resistance monitoring using soft μFBG group. (a) Schematic diagram of peripheral resistance monitoring. An inflatable cuff is used to impose the external pressure on the artery. The BCG signal and pulse wave at the RA site are continuously detected to calculate the PTT. (b) BCG signal (blue) and pulse wave (yellow) detected simultaneously by μFBG group. (c) PTT of every cardiac cycle (green dot) calculated from the vital signal. The trend of PTT indicated by the red line is calculated by moving averaging. The compression periods are indicated by the green region.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: