| Citation: | Li WB, Long YK, Yan YY et al. Wearable photonic smart wristband for cardiorespiratory function assessment and biometric identification. Opto-Electron Adv 8, 240254 (2025). doi: 10.29026/oea.2025.240254 |
Wearable photonic smart wristband for cardiorespiratory function assessment and biometric identification
-
Abstract
Personalized health services are of paramount importance for the treatment and prevention of cardiorespiratory diseases, such as hypertension. The assessment of cardiorespiratory function and biometric identification (ID) is crucial for the effectiveness of such personalized health services. To effectively and accurately monitor pulse wave signals, thus achieving the assessment of cardiorespiratory function, a wearable photonic smart wristband based on an all-polymer sensing unit (All-PSU) is proposed. The smart wristband enables the assessment of cardiorespiratory function by continuously monitoring respiratory rate (RR), heart rate (HR), and blood pressure (BP). Furthermore, it can be utilized for biometric ID purposes. Through the analysis of pulse wave signals using power spectral density (PSD), accurate monitoring of RR and HR is achieved. Additionally, utilizing peak detection algorithms for feature extraction from pulse signals and subsequently employing a variety of machine learning methods, accurate BP monitoring and biometric ID have been realized. For biometric ID, the accuracy rate is 98.55%. Aiming to monitor RR, HR, BP, and ID, our solution demonstrates advantages in integration, functionality, and monitoring precision. These enhancements may contribute to the development of personalized health services aimed at the treatment and prevention of cardiorespiratory diseases. -
-
References
[1] Timmis A, Vardas P, Townsend N et al. European society of cardiology: cardiovascular disease statistics 2021. Eur Heart J 43, 716–799 (2022). doi: 10.1093/eurheartj/ehab892 [2] Pelliccia A, Sharma S, Gati S et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: the Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 42, 17–96 (2021). doi: 10.1093/eurheartj/ehaa605 [3] Lytras T, Mouratidou E, Andreopoulou A et al. Effect of early oseltamivir treatment on mortality in critically ill patients with different types of influenza: a multiseason cohort study. Clin Infect Dis 69, 1896–1902 (2019). doi: 10.1093/cid/ciz101 [4] Torres A, Cilloniz C, Niederman MS et al. Pneumonia. Nat Rev Dis Primers 7, 25 (2021). doi: 10.1038/s41572-021-00259-0 [5] Lee JJ, Sundar KM. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung 199, 87–101 (2021). doi: 10.1007/s00408-021-00426-w [6] Fuster V. High blood pressure guidelines: welcomed advice, but let’s not lose the patient amid the numbers. J Am Coll Cardiol 71, 800–801 (2018). doi: 10.1016/j.jacc.2018.01.002 [7] Zhang XK, Wang C, He DD et al. Identification of DNA methylation-regulated genes as potential biomarkers for coronary heart disease via machine learning in the Framingham Heart Study. Clin Epigenetics 14, 122 (2022). doi: 10.1186/s13148-022-01343-2 [8] Couzin-Frankel J. Anti-inflammatory prevents heart attacks: by vindicating theory, antibody result points to new approaches to protecting the heart. Science 357, 855 (2017). doi: 10.1126/science.357.6354.855 [9] Girard M, Deschamps J, Razzaq S et al. Emerging applications of extracardiac ultrasound in critically ill cardiac patients. Can J Cardiol 39, 444–457 (2023). doi: 10.1016/j.cjca.2022.11.015 [10] Pujol-López M, San Antonio R, Mont L et al. Electrocardiographic optimization techniques in resynchronization therapy. EP Eur 21, 1286–1296 (2019). [11] Zhang J, Fletcher JG, Harmsen WS et al. Analysis of heart rate and heart rate variation during cardiac CT examinations. Acad Radiol 15, 40–48 (2008). doi: 10.1016/j.acra.2007.07.023 [12] Meng KY, Xiao X, Wei WX et al. Wearable pressure sensors for pulse wave monitoring. Adv Mater 34, 2109357 (2022). doi: 10.1002/adma.202109357 [13] Mohapatra D, Byun JE, Ansari MZ et al. Layer engineered MXene empowered wearable pressure sensors for non‐invasive vital human–machine interfacing healthcare monitoring. Adv Mater Technol 8, 2301175 (2023). doi: 10.1002/admt.202301175 [14] Meng KY, Xiao X, Liu ZX et al. Kirigami‐inspired pressure sensors for wearable dynamic cardiovascular monitoring. Adv Mater 34, 2202478 (2022). doi: 10.1002/adma.202202478 [15] Xiang ZH, Han MD, Zhang HX. Nanomaterials based flexible devices for monitoring and treatment of cardiovascular diseases (CVDs). Nano Res 16, 3939–3955 (2023). doi: 10.1007/s12274-022-4551-8 [16] Han LY, Liang WJ, Xie QS et al. Health monitoring via heart, breath, and korotkoff sounds by wearable piezoelectret patches. Adv Sci 10, 2301180 (2023). doi: 10.1002/advs.202301180 [17] Shi X, Zuo Y, Zhai P et al. Large-area display textiles integrated with functional systems. Nature 591, 240–245 (2021). doi: 10.1038/s41586-021-03295-8 [18] Lee S, Franklin S, Hassani FA et al. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 370, 966–970 (2020). doi: 10.1126/science.abc9735 [19] Jiang YW, Zhang ZT, Wang YX et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022). doi: 10.1126/science.abj7564 [20] Wang DS, Li JP, Memik G. User identification based on finger-vein patterns for consumer electronics devices. IEEE Trans Consum Electron 56, 799–804 (2010). doi: 10.1109/TCE.2010.5506004 [21] Xu W, Liu SD, Yang JY et al. Self-powered flexible handwriting input panel with 1D output enabled by convolutional neural network. Nano Energy 101, 107557 (2022). doi: 10.1016/j.nanoen.2022.107557 [22] Maddirala G, Searle T, Wang X et al. Multifunctional skin-compliant wearable sensors for monitoring human condition applications. Appl Mater Today 26, 101361 (2022). doi: 10.1016/j.apmt.2021.101361 [23] Kireev D, Sel K, Ibrahim B et al. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat Nanotechnol 17, 864–870 (2022). doi: 10.1038/s41565-022-01145-w [24] Li Z, Chen JX, Li LZ et al. Exceptional-point-enhanced sensing in an all-fiber bending sensor. Opto-Electron Adv 6, 230019 (2023). doi: 10.29026/oea.2023.230019 [25] Feng HG, Chen X, Zhu RZ et al. Seeing at a distance with multicore fibers. Opto-Electron Adv 7, 230202 (2024). doi: 10.29026/oea.2024.230202 [26] Liu HH, Hu DJJ, Sun QZ et al. Specialty optical fibers for advanced sensing applications. Opto-Electron Sci 2, 220025 (2023). doi: 10.29026/oes.2023.220025 [27] Cai ZH, Li BZ, Bai ZY et al. Encrypted optical fiber tag based on encoded fiber Bragg grating array. Int J Extreme Manuf 5, 035502 (2023). doi: 10.1088/2631-7990/acd825 [28] Zhang L, Zhen YQ, Tong LM. Optical micro/nanofiber enabled tactile sensors and soft actuators: a review. Opto-Electron Sci 3, 240005 (2024). doi: 10.29026/oes.2024.240005 [29] Pan J, Wang Q, Gao SK et al. Knot-inspired optical sensors for slip detection and friction measurement in dexterous robotic manipulation. Opto-Electron Adv 6, 230076 (2023). doi: 10.29026/oea.2023.230076 [30] Jha R, Mishra P, Kumar S. Advancements in optical fiber-based wearable sensors for smart health monitoring. Biosens Bioelectron 254, 116232 (2024). doi: 10.1016/j.bios.2024.116232 [31] Min R, Hu XH, Pereira L et al. Polymer optical fiber for monitoring human physiological and body function: a comprehensive review on mechanisms, materials, and applications. Opt Laser Technol 147, 107626 (2022). doi: 10.1016/j.optlastec.2021.107626 [32] Guo JJ, Zhou BQ, Zong R et al. Stretchable and highly sensitive optical strain sensors for human-activity monitoring and healthcare. ACS Appl Mater Interfaces 11, 33589–33598 (2019). doi: 10.1021/acsami.9b09815 [33] Zhu HT, Luo JX, Dai Q et al. Spatiotemporal hemodynamic monitoring via configurable skin-like microfiber Bragg grating group. Opto-Electron Adv 6, 230018 (2023). doi: 10.29026/oea.2023.230018 [34] Chen MH, He YC, Liang HH et al. Stretchable and strain-decoupled fluorescent optical fiber sensor for body temperature and movement monitoring. ACS Photonics 9, 1415–1424 (2022). doi: 10.1021/acsphotonics.2c00249 [35] Tan FZ, Lyu WM, Chen SY et al. Contactless vital signs monitoring based on few-mode and multi-core fibers. Opto-Electron Adv 3, 190034 (2020). doi: 10.29026/oea.2020.190034 [36] Guo JJ, Niu MX, Yang CX. Highly flexible and stretchable optical strain sensing for human motion detection. Optica 4, 1285–1288 (2017). doi: 10.1364/OPTICA.4.001285 [37] Li LY, Liu YF, Song CY et al. Wearable alignment-free microfiber-based sensor chip for precise vital signs monitoring and cardiovascular assessment. Adv Fiber Mater 4, 475–486 (2022). doi: 10.1007/s42765-021-00121-8 [38] Zhu HT, Zhan LW, Dai Q et al. Self‐assembled wavy optical microfiber for stretchable wearable sensor. Adv Opt Mater 9, 2002206 (2021). doi: 10.1002/adom.202002206 [39] Pang YN, Liu B, Liu J et al. Singlemode-multimode-singlemode optical fiber sensor for accurate blood pressure monitoring. J Light Technol 40, 4443–4450 (2022). doi: 10.1109/JLT.2022.3155194 [40] Li LY, Sheng SF, Liu YF et al. Automatic and continuous blood pressure monitoring via an optical-fiber-sensor-assisted smartwatch. PhotoniX 4, 21 (2023). doi: 10.1186/s43074-023-00099-z [41] Wang YK, Yu XL, Jiang CL et al. Micro-nano fiber flexible multimodal sensors for fingerprint recognition. IEEE Sens J 24, 4504–4509 (2024). doi: 10.1109/JSEN.2023.3347201 [42] Li JH, Chen L, Yan XP et al. Super-stretchable polymer optical fibers for robot finger posture and pressure recognition. J Light Technol (2024), doi: 10.1109/JLT.2024.3519531. [43] Wang Z, Chen ZY, Ma L et al. Optical microfiber intelligent sensor: wearable cardiorespiratory and behavior monitoring with a flexible wave-shaped polymer optical microfiber. ACS Appl Mater Interfaces 16, 8333–8345 (2024). doi: 10.1021/acsami.3c16165 [44] Kuang RF, Wang Z, Ma L et al. Smart photonic wristband for pulse wave monitoring. Opto-Electron Sci 3, 240009 (2024). doi: 10.29026/oes.2024.240009 [45] Leal-Junior A, Avellar L, Biazi V et al. Multifunctional flexible optical waveguide sensor: on the bioinspiration for ultrasensitive sensors development. Opto-Electron Adv 5, 210098 (2022). doi: 10.29026/oea.2022.210098 [46] Wang LL, Zhong C, Ke DN et al. Ultrasoft and highly stretchable hydrogel optical fibers for in vivo optogenetic modulations. Adv Opt Mater 6, 1800427 (2018). doi: 10.1002/adom.201800427 [47] Zha BJ, Wang Z, Ma L et al. Intelligent wearable photonic sensing system for remote healthcare monitoring using stretchable elastomer optical fiber. IEEE Internet Things J 11, 17317–17329 (2024). doi: 10.1109/JIOT.2024.3356574 [48] Rendeiro R, Jargus J, Nedoma J et al. The possibilities of using a mixture of PDMS and phosphor in a wide range of industry applications. Opto-Electron Adv 7, 240133 (2024). doi: 10.29026/oea.2024.240133 [49] Jakubowski K, Huang CS, Boesel LF et al. Recent advances in photoluminescent polymer optical fibers. Curr Opin Solid State Mater Sci 25, 100912 (2021). doi: 10.1016/j.cossms.2021.100912 [50] Li TL, Wang QA, Su YF et al. AI-assisted disease monitoring using stretchable polymer-based sensors. ACS Appl Mater Interfaces 15, 30924–30934 (2023). doi: 10.1021/acsami.3c01970 [51] Fan XY, Huang Y, Ding XR et al. Alignment‐free liquid‐capsule pressure sensor for cardiovascular monitoring. Adv Funct Mater 28, 1805045 (2018). doi: 10.1002/adfm.201805045 [52] Fu Y, Zhao S, Wang LQ et al. A wearable sensor using structured silver‐particle reinforced PDMS for radial arterial pulse wave monitoring. Adv Healthc Mater 8, 1900633 (2019). doi: 10.1002/adhm.201900633 [53] Song ZQ, Li WY, Bao Y et al. Bioinspired microstructured pressure sensor based on a janus graphene film for monitoring vital signs and cardiovascular assessment. Adv Electron Mater 4, 1800252 (2018). doi: 10.1002/aelm.201800252 [54] Li YJ, Wang ZL, Zhang L et al. Characters available in photoplethysmogram for blood pressure estimation: beyond the pulse transit time. Australas Phys Eng Sci Med 37, 367–376 (2014). doi: 10.1007/s13246-014-0269-6 [55] Ding M, Zhou H, Xie H et al. A gated recurrent unit neural networks based wind speed error correction model for short-term wind power forecasting. Neurocomputing 365, 54–61 (2019). doi: 10.1016/j.neucom.2019.07.058 [56] Wang YS, Xia ST, Tang QT et al. Novel consistent random forest framework: Bernoulli random forests. IEEE Trans Neural Netw Learn Syst 29, 3510–3523 (2018). doi: 10.1109/TNNLS.2017.2729778 [57] Liu YC, Li HY, Liang XP et al. Speech recognition using intelligent piezoresistive sensor based on polystyrene sphere microstructures. Adv Intell Syst 5, 2200427 (2023). doi: 10.1002/aisy.202200427 [58] Yu ZC, Xu JH, Gong HX et al. Bioinspired self-powered piezoresistive sensors for simultaneous monitoring of human health and outdoor UV light intensity. ACS Appl Mater Interfaces 14, 5101–5111 (2022). doi: 10.1021/acsami.1c23604 [59] Mizuno R, Fujimoto S, Nakano H et al. Atrial conduction abnormalities in patients with systemic progressive sclerosis. Eur Heart J 18, 1995–2001 (1997). doi: 10.1093/oxfordjournals.eurheartj.a015211 [60] Mejía-Mejía E, May JM, Elgendi M et al. Differential effects of the blood pressure state on pulse rate variability and heart rate variability in critically ill patients. Npj Digit Med 4, 82 (2021). doi: 10.1038/s41746-021-00447-y [61] Favilla R, Zuccalà VC, Coppini G. Heart rate and heart rate variability from single-channel video and ICA integration of multiple signals. IEEE J Biomed Health Inform 23, 2398–2408 (2019). doi: 10.1109/JBHI.2018.2880097 [62] Peetermans M, Guler I, Meersseman P et al. Impact of BMI on outcomes in respiratory ECMO: an ELSO registry study. Intensive Care Med 49, 37–49 (2023). doi: 10.1007/s00134-022-06926-4 [63] Boehmer J, Mark G, Wen GZ et al. Impact of body mass index on device measured diagnostic sensor measurements in ambulatory heart failure patients. J Card Fail 24, S128–S129 (2018). [64] Guo X, Wang Y, Zhou NW et al. Optimal weighted two-sample t-test with partially paired data in a unified framework. J Appl Stat 48, 961–976 (2021). doi: 10.1080/02664763.2020.1753027 [65] Dominelli PB, Archiza B, Ramsook AH et al. Effects of respiratory muscle work on respiratory and locomotor blood flow during exercise. Exp Physiol 102, 1535–1547 (2017). doi: 10.1113/EP086566 [66] Chan PY, Ryan NP, Chen D et al. Novel wearable and contactless heart rate, respiratory rate, and oxygen saturation monitoring devices: a systematic review and meta‐analysis. Anaesthesia 77, 1268–1280 (2022). doi: 10.1111/anae.15834 [67] Luo H, Yang DY, Barszczyk A et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging 12, e008857 (2019). doi: 10.1161/CIRCIMAGING.119.008857 [68] Hermányi Z, Pokoly B, Visolyi G et al. Evaluation of meditech ABPM-06 ambulatory blood pressure measuring device, according to the European Society of Hypertension, the British Hypertension Society and the International Organization for Standardization protocol. Blood Press Monit 24, 208–211 (2019). doi: 10.1097/MBP.0000000000000385 [69] Debray A, Ravanelli N, Chenette-Stewart O et al. Effect of exercise training on blood pressure in healthy postmenopausal females: a systematic review with meta-analysis. Med Sci Sports Exerc 55, 1317–1325 (2023). doi: 10.1249/MSS.0000000000003142 -
Supplementary Information
Supplementary information for Wearable photonic smart wristband for cardiorespiratory function assessment and biometric identification 
-
Access History
Article Metrics
-
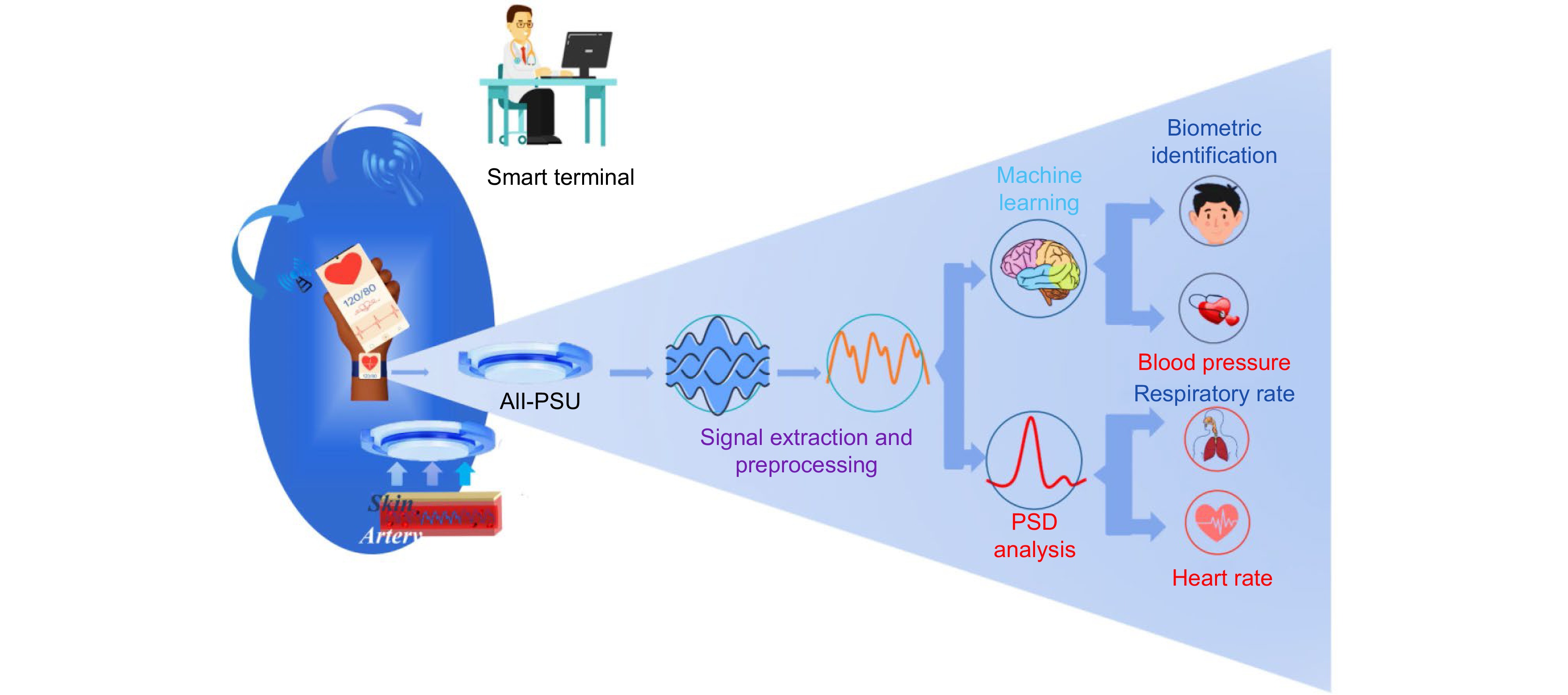
Figure 1.
Functional architecture of the proposed wristband, including mainly five primary components: the ALL-PSU sensing unit, signal extraction and preprocessing, machine learning models, and PSD analysis.
-
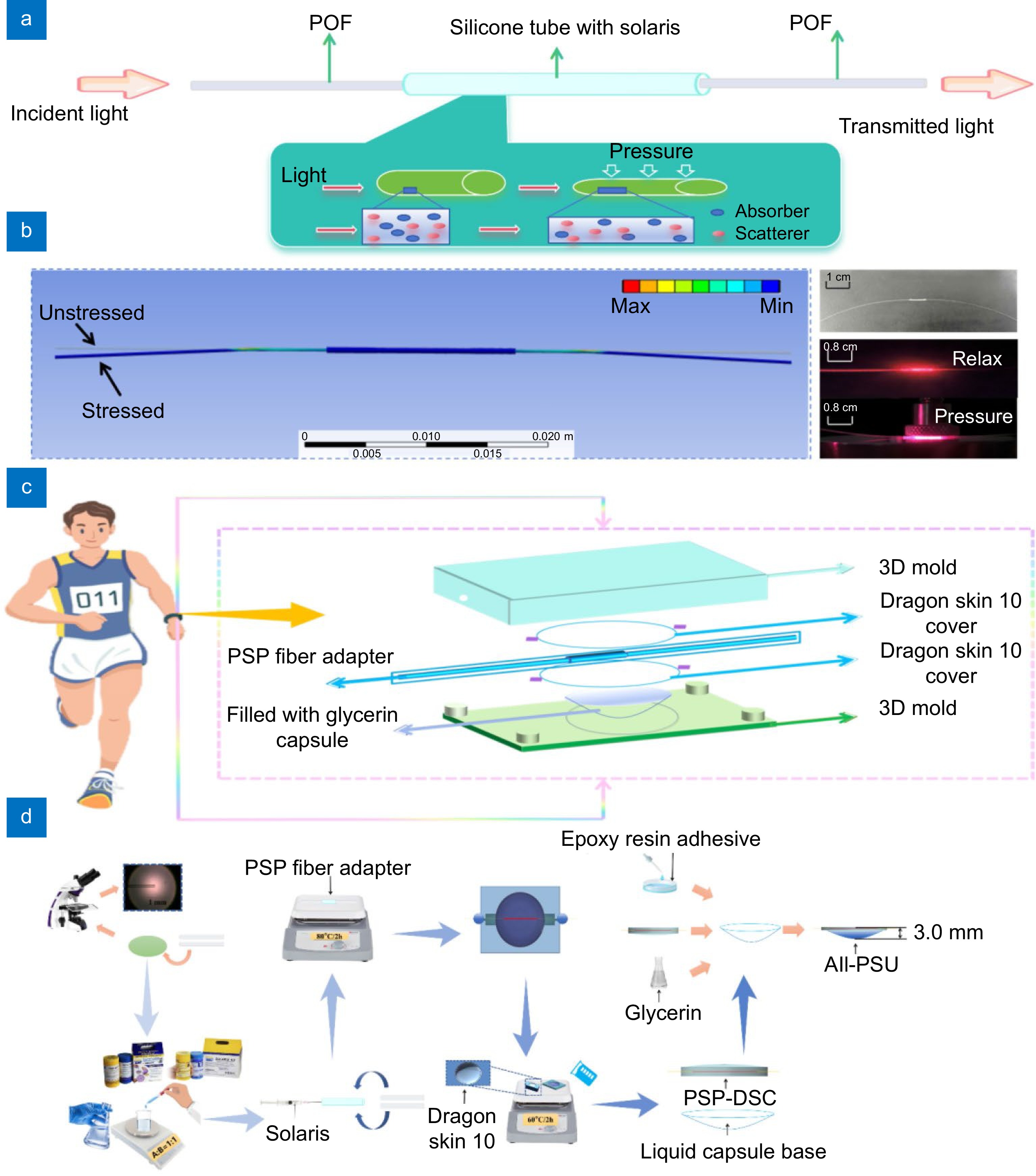
Figure 2.
(a) The conceptual figure of the PSP fiber adapter. (b) Pressure simulation (left) alongside the actual configuration of the PSP fiber adapter (right), demonstrating the adapter's response to pressure. (c) Exploded schematic of the practical All-PSU, including 3D molds, DSCs, PSP fiber adapter, and a glycerol-filled capsule. (d) Structural diagram of the All-PSU, the entire process of All-PSU is divided into three parts: the PSP fiber adapter fabrication, the PSP-DSC and liquid capsule base fabrication, and the All-PSU construction and coating.
-
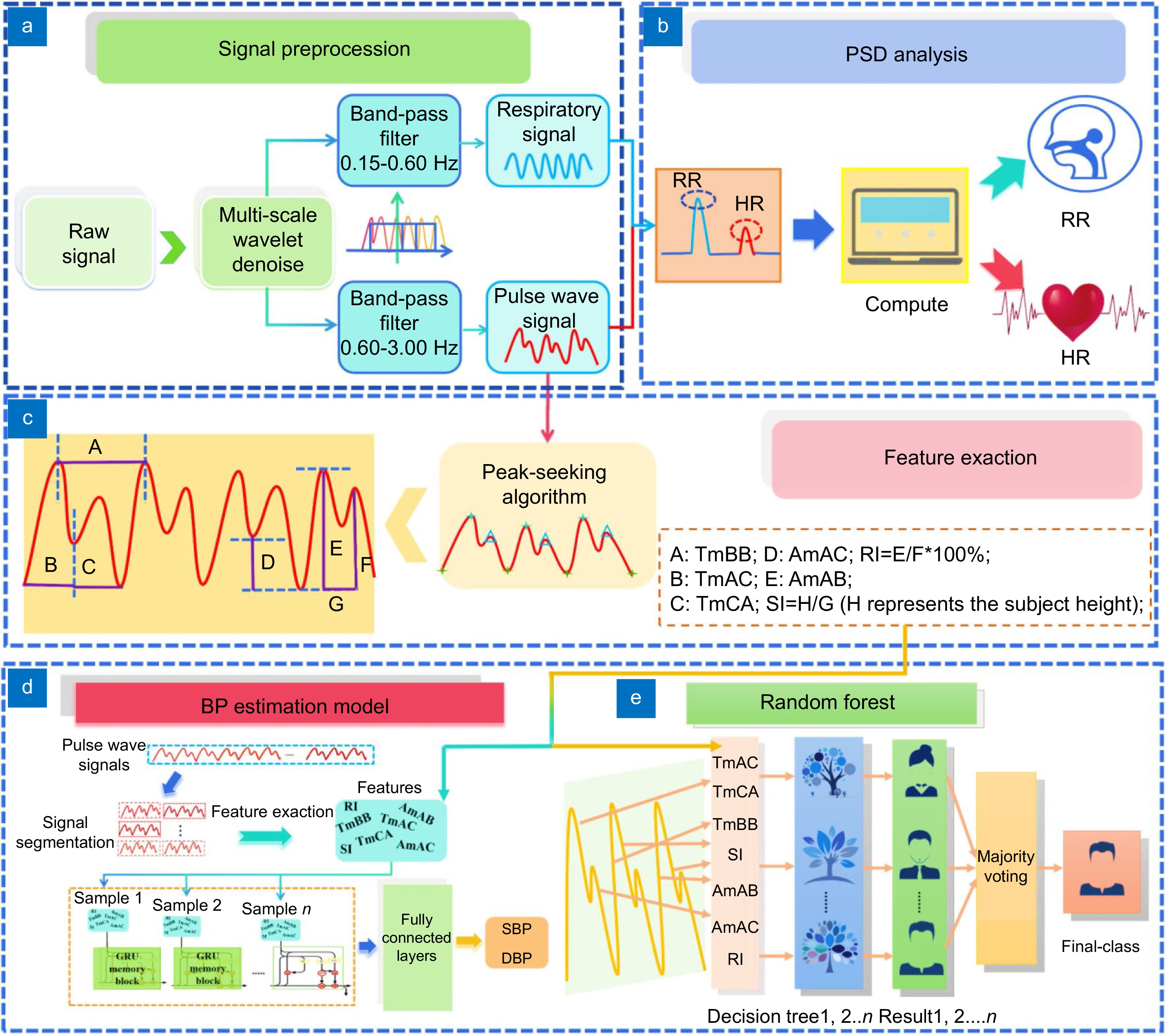
Figure 3.
(a) Method to extract clean and accurate pulse wave signals as well as respiratory signals. (b) PSD analysis method utilized to monitor RR and HR. (c) Feature extraction process and the seven distinct features extracted from the pulse wave signals, which are utilized for both BP estimation and biometric ID (H represents the subject height). (d) Architecture of the GRU model developed for BP estimation. (e) Structure of the RF classifier implemented for biometric ID.
-
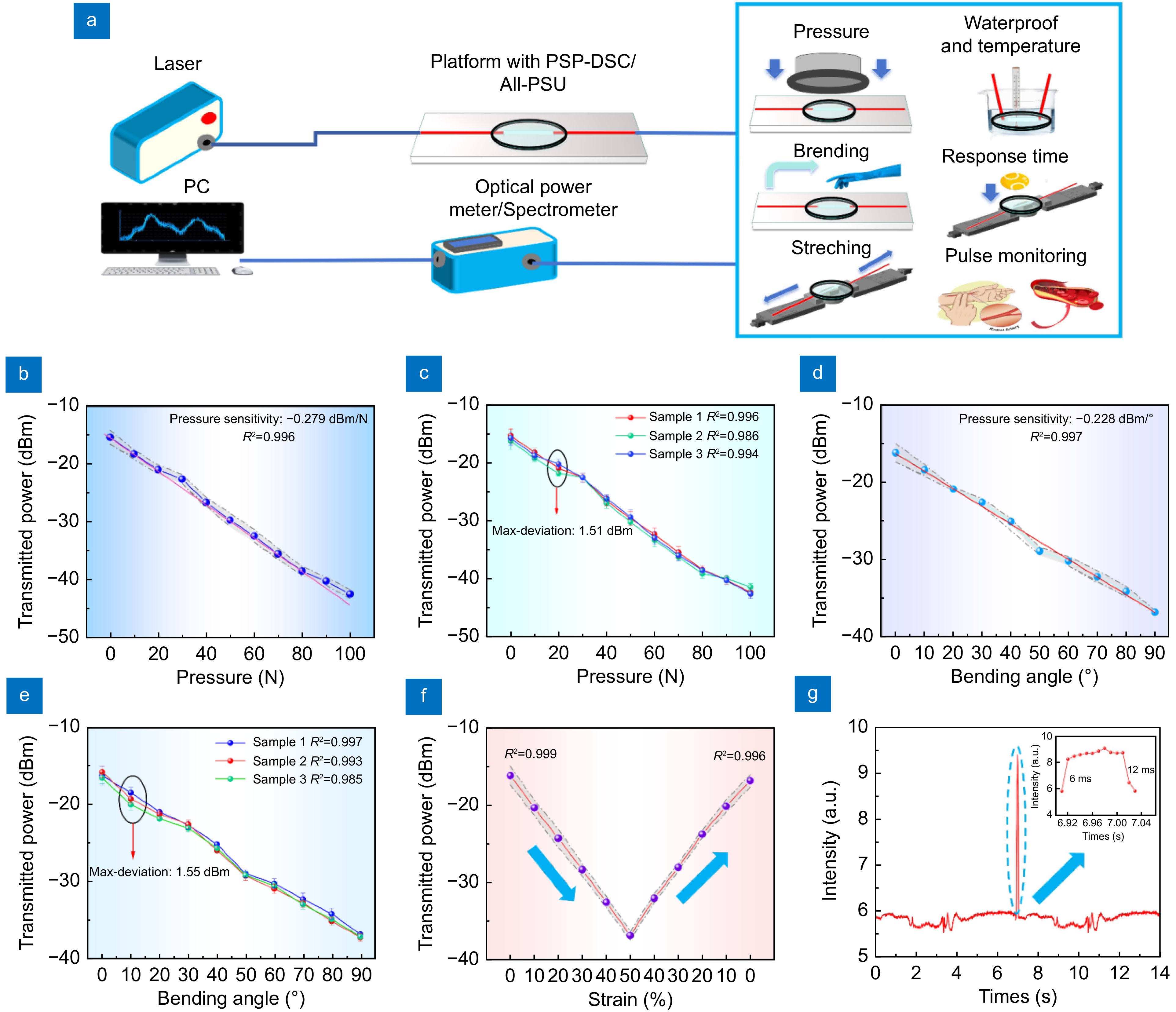
Figure 4.
(a) Schematic diagram of the PSP-DSC and All-PSU performance test experiments. (b) Transmitted power of PSP-DSC under varying pressures, with pressure increased by 10 N each time. The line plot illustrates the mean value of the transmitted light power derived from the three repeated experiments. The gray area surrounding the line plot represents the data distribution across these trials. (This applies similarly to subfigure (d) and (f)). (c) Transmitted power of three PSP-DSC samples under varying pressures, with pressure increased by 10 N each time. (d) Transmitted power of PSP-DSC under different bending angles, with angles increased by 10° each time. (e) Transmitted power of three PSP-DSC samples under different bending angles, with angles increased by 10° each time. (f) Transmitted light power of PSP-DSC under tensile strains, with deformation increased by 10% every 30 seconds. (g) Transient pulse response time results (response time: 6 ms, recovery time: 12 ms).
-
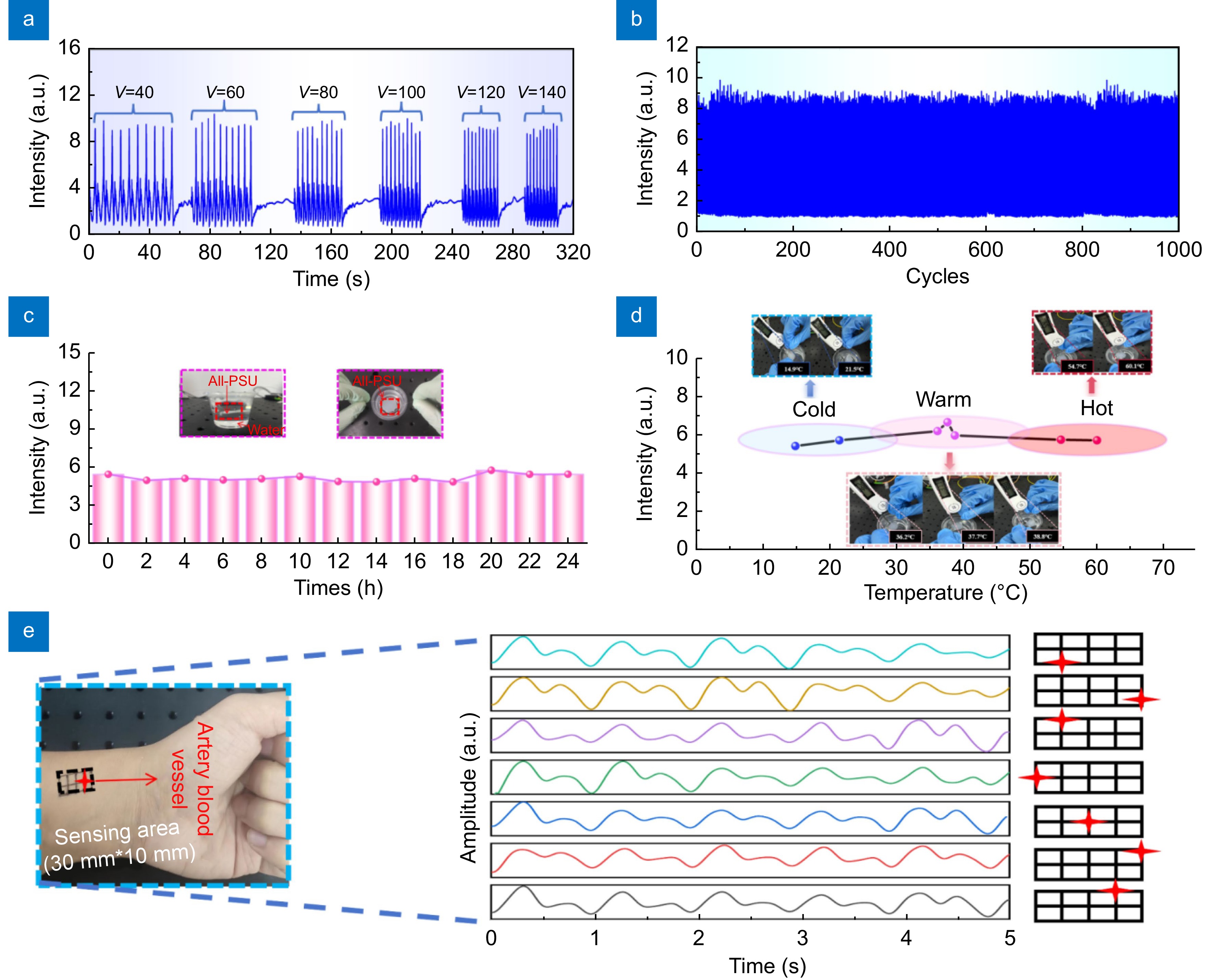
Figure 5.
(a) The dynamic response of the All-PSU. Evaluated across a range of tensile velocities on stepper motors. (b) Performance assessment of the All-PSU through cyclic tensile strain testing, subjected to a tensile strain of 50% over 1,000 cycles. (c) Waterproofing of the All-PSU confirmed by immersion in water at room temperature (23 °C) for 24 hours, with response intensity recorded every 2 hours. (d) Response intensity of the All-PSU placed in water at various temperature. (e) Pulse wave signal monitoring at different wrist locations, with the star shape indicating the position of the All-PSU.
-
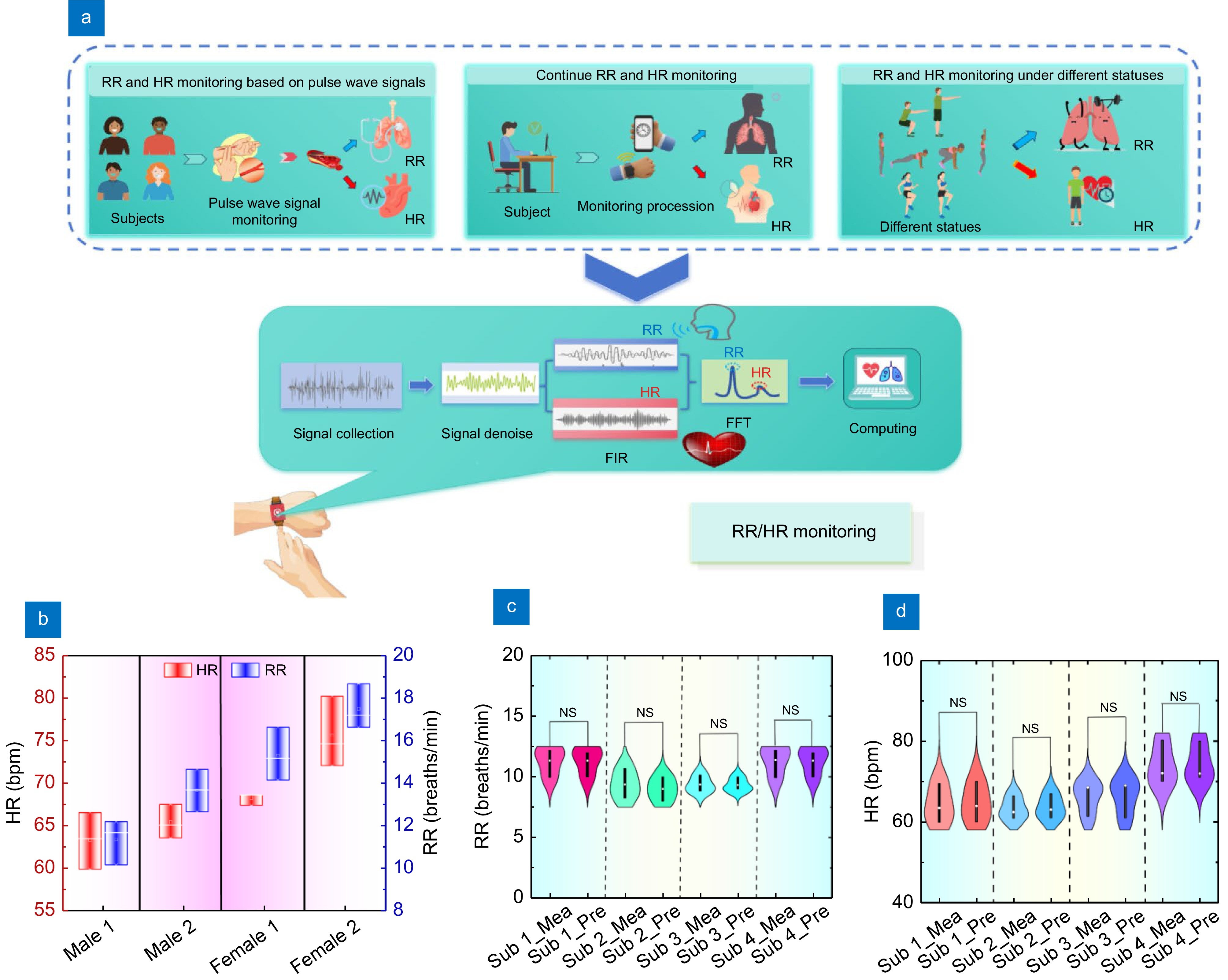
Figure 6.
(a) Diagram and processing schematic of RR and HR monitoring system based on PSD analysis. The experiment was mainly divided into three stages: four subjects monitored their RR and HR under relaxed conditions, another continuously monitored his RR and HR from 14:00–18:00, and four subjects monitored their RR and HR under different states (squat. burpee and high knee). Signal processing is mainly divided into signal acquisition, signal noise reduction, signal extraction, frequency domain conversion, and RR and HR calculation. (b) Line box plot of the four subject's results of RR and HR for four subjects. (c) Violin plot of the four subjects measured and predicted RR results, and (d) Violin plot of the four subjects measured and predicted HR results. NS: indicates that there is no significant difference between the results of measured and predicted, indicating that the results of predicted HR, according to the proposed system, are accurate for different subjects.
-
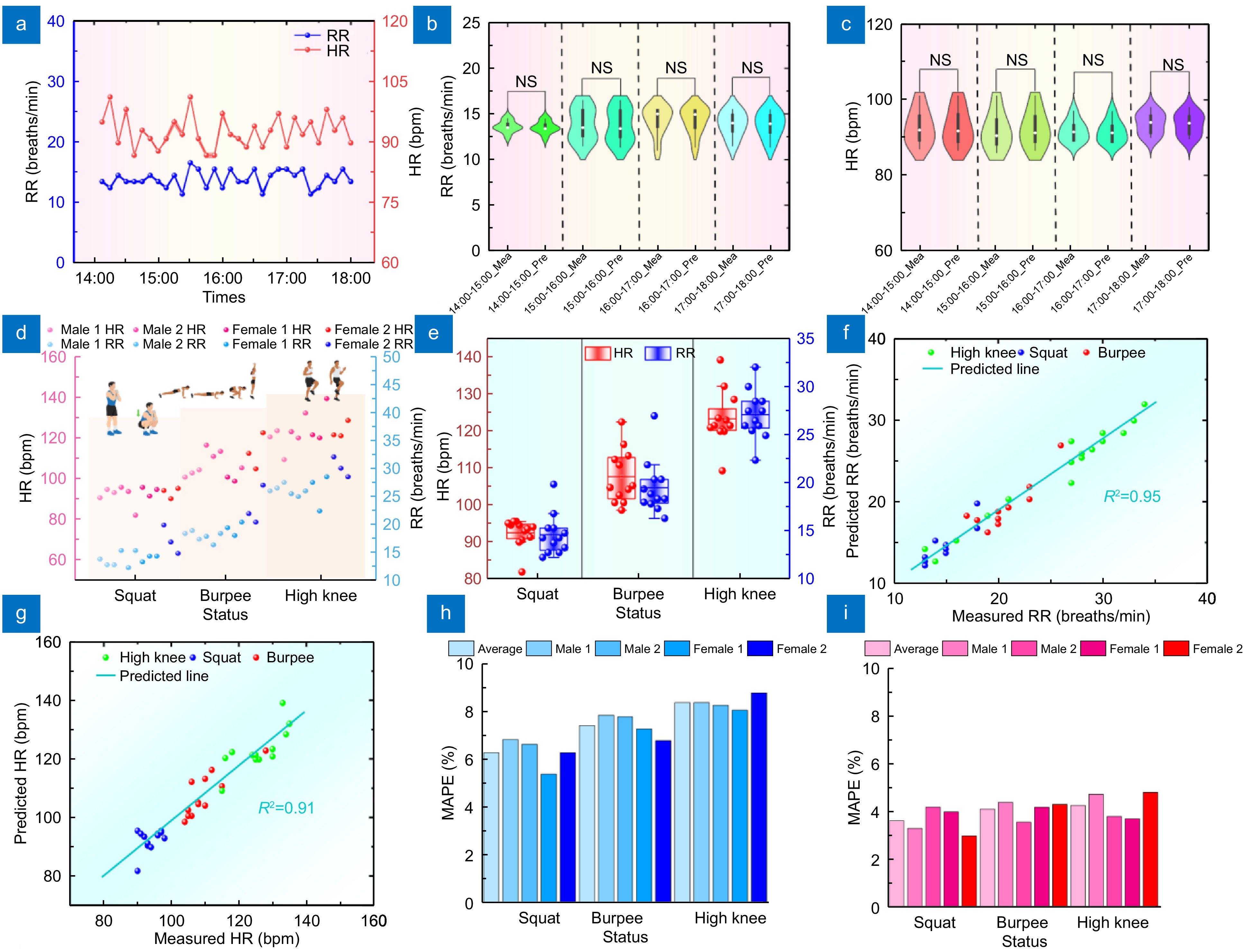
Figure 7.
(a) RR and HR monitoring results at 14:00–18:00. (b) Violin plot comparing measured and reference RR results during 14:00–18:00, showing no significant difference and suggesting accurate continuous RR measurement. (c) Violin plot comparing measured and reference HR results during 14:00–18:00, showing no significant difference and suggesting accurate continuous HR measurement. (d) Scatter plot of RR and HR monitoring results of four subjects in squatting, burpee, and high knee. (e) Line box plot of RR and HR monitoring results of subjects in different statuses. (f) Correlation result plot between predicted and measured RR in different exercises. (g) Correlation result plots between the predicted and measured HR in different exercise states. (h) MAPE plot of RR predictions for subjects in different exercise states. (i) MAPE Plot of HR predictions of four subjects in different exercise states.
-
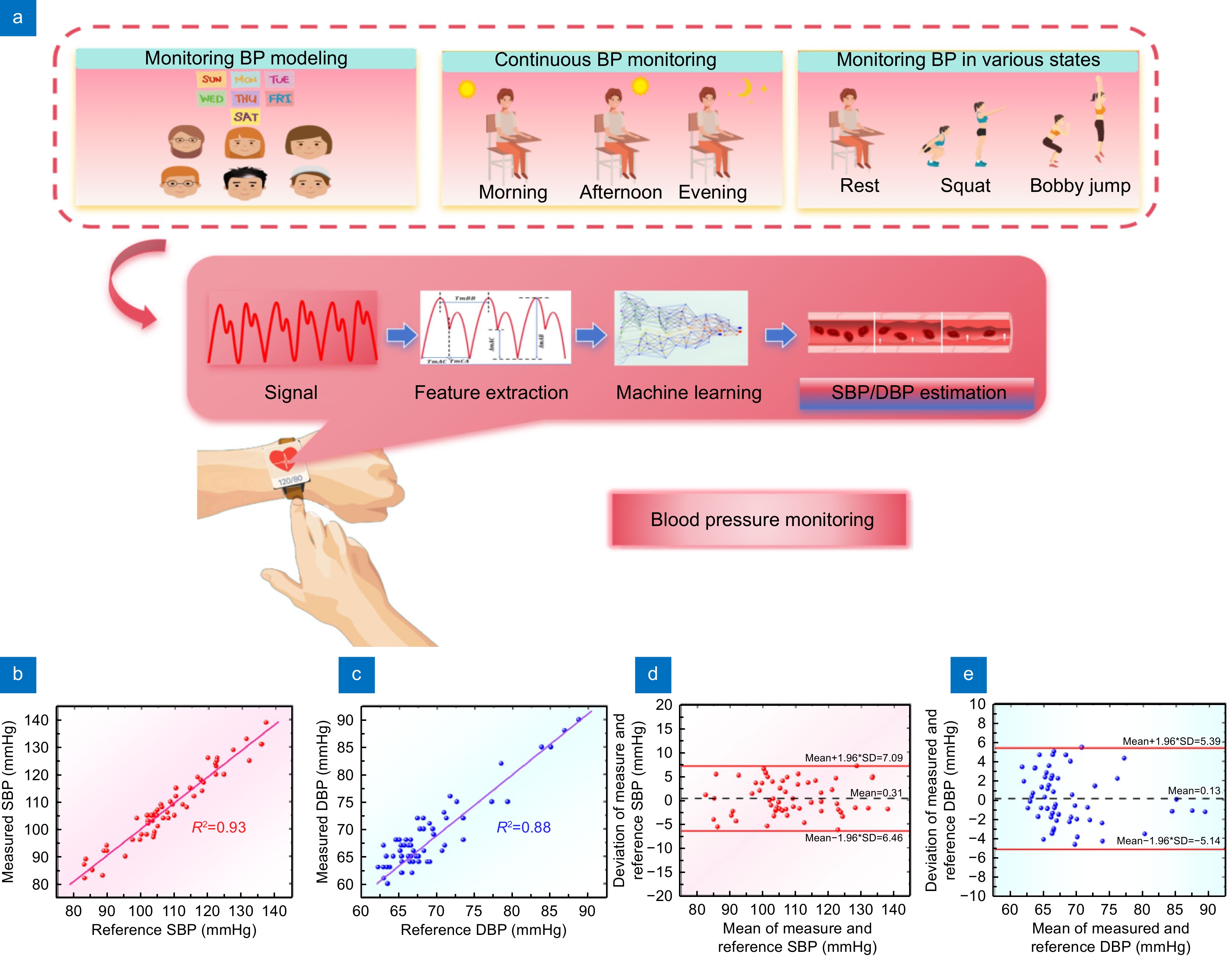
Figure 8.
(a) Schematic diagram of three pivotal phases and BP estimation model processing the whole BP monitoring process is divided into six subjects to continuously monitor BP for one week to establish a model, another subject monitored BP changes in the morning, afternoon, and evening for one day, and another subject monitored BP fluctuations in different states (resting squat, burpee), the main model processing is divided into signal acquisition, feature extraction, and machine learning model estimation. (b) Correlation analysis graph between SBP measured results and reference results. (c) Correlation analysis plot of DBP measured results and reference results. (d) Bland-Altman plots of SBP, and (e) Bland-Altman plots of DBP.
-
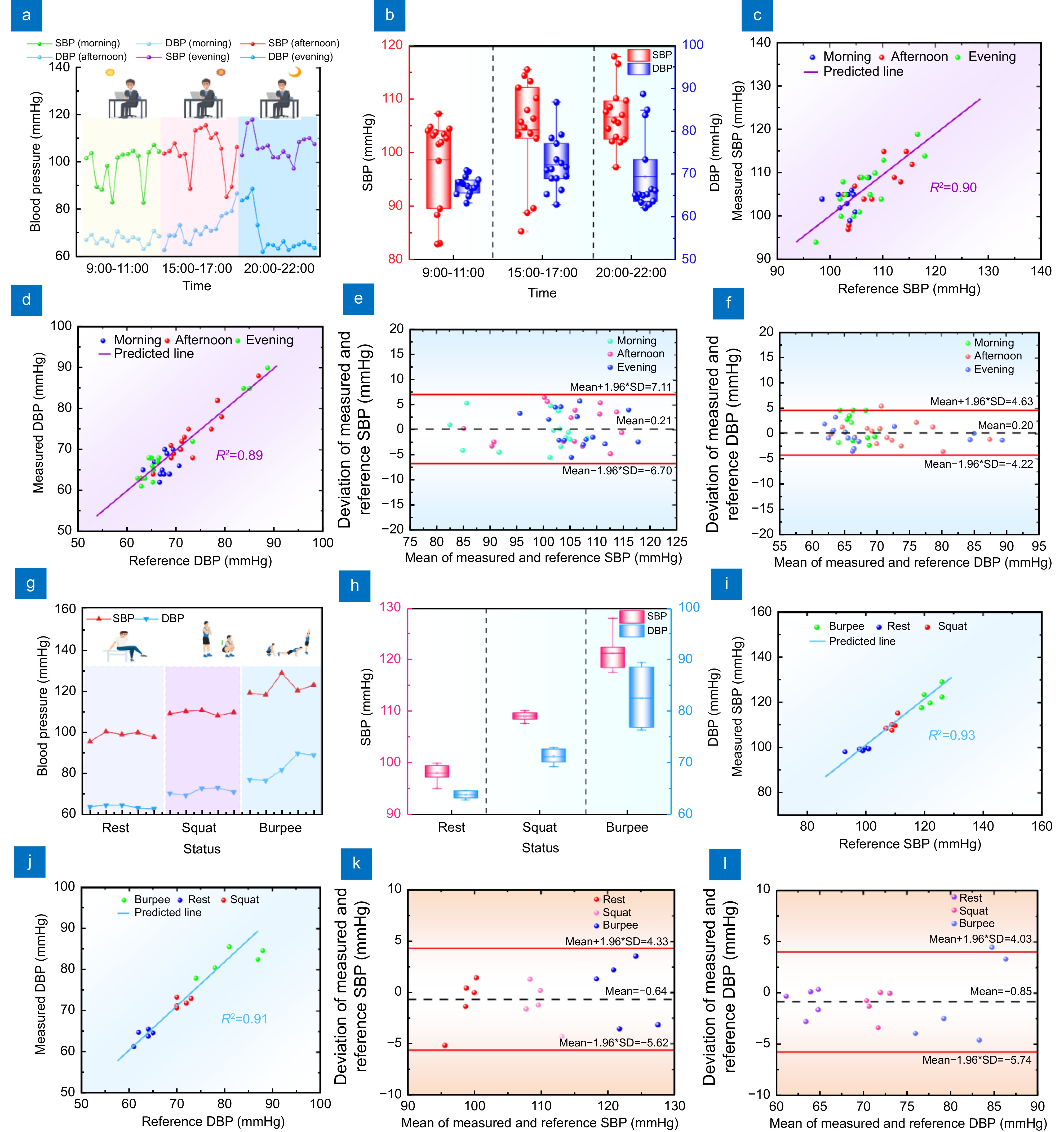
Figure 9.
(a) Line graph of BP measurement results in the morning, afternoon, and evening. (b) Line box plots of BP measured results in the morning, afternoon, and evening. (c) Correlation analysis of SBP measured and reference results. (d) Correlation analysis of DBP measured and reference results. (e) Bland-Altman plot of SBP in the morning, afternoon, and evening. (f) Bland-Altman plot of DBP in the morning, afternoon, and evening. (g) Line graph of BP measurement results at rest, squat, and jump. (h) Line box plot of BP measurements at rest, squat, and burpee. (i) Correlation analysis of SBP measured and reference results under different exercise statuses. (j) Correlation analysis of DBP measured and reference results under different exercise states. (k) Bland-Altman plot of SBP at rest, squat, and burpee. (l) Bland-Altman plot of DBP at rest, squat, and burpee.
-
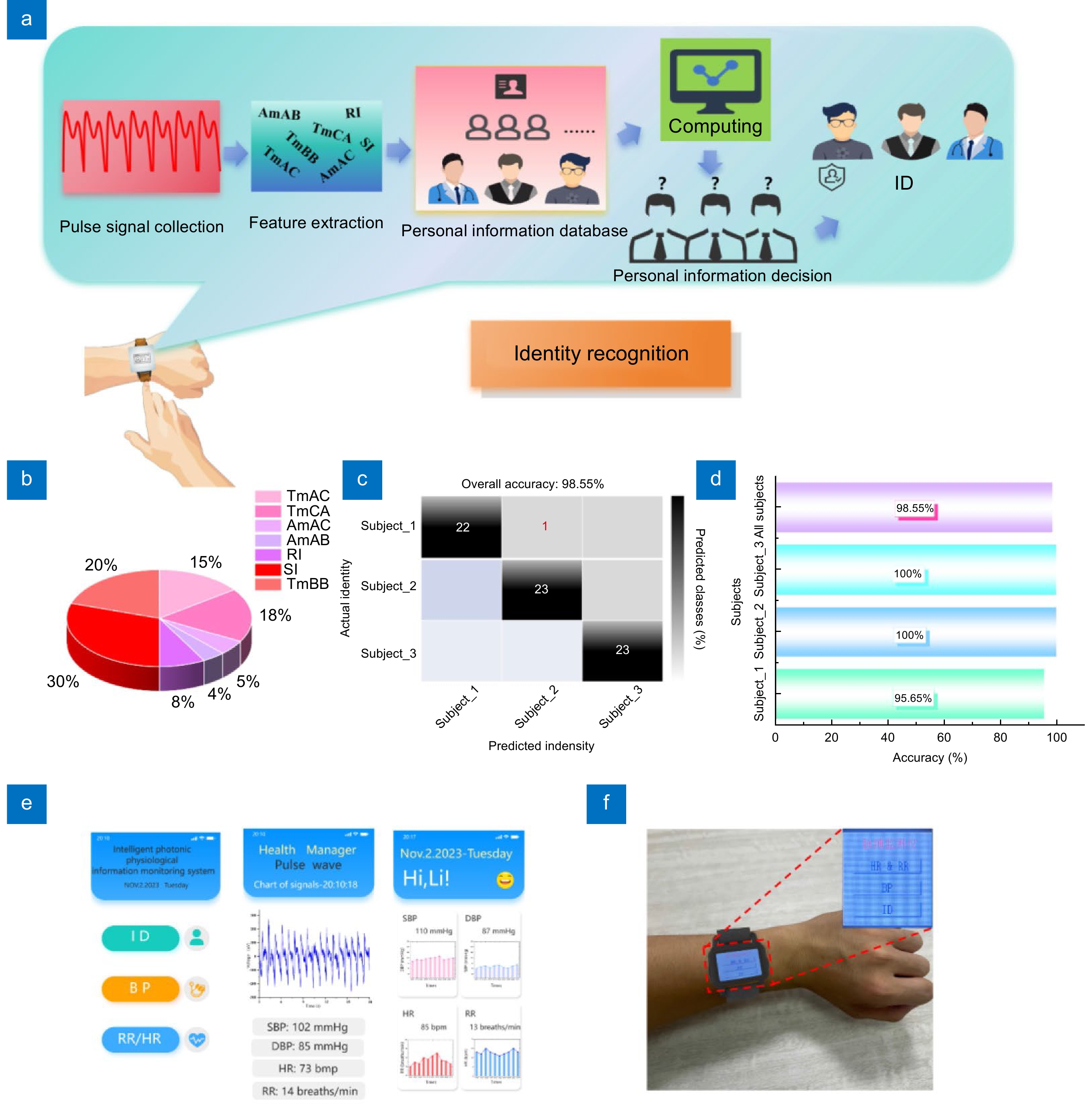
Figure 10.
(a) Schematic diagram of the biometric identification process, which mainly includes pulse signal collection, pulse feature extraction, personal information database establishment, RF algorithm decision-making, and identification results. (b) The weighting pie chart of the seven features in the biometric identification process. (c) Confusion matrix for biometric identification results. (d) Biometric identification accuracy of different types of subjects and total prediction accuracy of three subjects. (e) The customized APP, a personal information interface, a week historical data interface, and a real-time measurement interface, and (f) The smartwatch worn by a 24-year-old male.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: