| Citation: | Chernomyrdin NV, Musina GR, Nikitin PV, Dolganova IN, Kucheryavenko AS et al. Terahertz technology in intraoperative neurodiagnostics: A review. Opto-Electron Adv 6, 220071 (2023). doi: 10.29026/oea.2023.220071 |
Terahertz technology in intraoperative neurodiagnostics: A review
-
Abstract
Terahertz (THz) technology offers novel opportunities in biology and medicine, thanks to the unique features of THz-wave interactions with tissues and cells. Among them, we particularly notice strong sensitivity of THz waves to the tissue water, as a medium for biochemical reactions and a main endogenous marker for THz spectroscopy and imaging. Tissues of the brain have an exceptionally high content of water. This factor, along with the features of the structural organization and biochemistry of neuronal and glial tissues, makes the brain an exciting subject to study in the THz range. In this paper, progress and prospects of THz technology in neurodiagnostics are overviewed, including diagnosis of neurodegenerative disease, myelin deficit, tumors of the central nervous system (with an emphasis on brain gliomas), and traumatic brain injuries. Fundamental and applied challenges in study of the THz-wave – brain tissue interactions and development of the THz biomedical tools and systems for neurodiagnostics are discussed. -
-
References
[1] Lee YS. Principles of Terahertz Science and Technology (Springer, New York, NY, USA, 2009). [2] Guerboukha H, Nallappan K, Skorobogatiy M. Toward real-time terahertz imaging. Adv Opt Photonics 10, 843–938 (2018). doi: 10.1364/AOP.10.000843 [3] Yachmenev AE, Lavrukhin DV, Glinskiy IA, Zenchenko NV, Goncharov YG et al. Metallic and dielectric metasurfaces in photoconductive terahertz devices: a review. Opt Eng 59, 061608 (2019). [4] Yachmenev AE, Pushkarev SS, Reznik RR, Khabibullin RA, Ponomarev DS. Arsenides-and related III-V materials-based multilayered structures for terahertz applications: various designs and growth technology. Prog Cryst Growth Charact Mater 66, 100485 (2020). doi: 10.1016/j.pcrysgrow.2020.100485 [5] Islam MS, Cordeiro CMB, Franco MAR, Sultana J, Cruz ALS et al. Terahertz optical fibers [invited]. Opt Express 28, 16089–16117 (2020). doi: 10.1364/OE.389999 [6] Katyba GM, Zaytsev KI, Dolganova IN, Chernomyrdin NV, Ulitko VE et al. Sapphire waveguides and fibers for terahertz applications. Prog Cryst Growth Charact Mater 67, 100523 (2021). doi: 10.1016/j.pcrysgrow.2021.100523 [7] Zaytsev KI, Katyba GM, Chernomyrdin NV, Dolganova IN, Kucheryavenko AS et al. Overcoming the abbe diffraction limit using a bundle of metal-coated high-refractive-index sapphire optical fibers. Adv Opt Mater 8, 2000307 (2020). doi: 10.1002/adom.202000307 [8] Rubens H, Nichols EF. Heat rays of great wave length. Phys Rev (Series I) 4, 314–323 (1897). doi: 10.1103/PhysRevSeriesI.4.314 [9] Auston DH. Picosecond optoelectronic switching and gating in silicon. Appl Phys Lett 26, 101–103 (1975). doi: 10.1063/1.88079 [10] Zaytsev KI, Dolganova IN, Chernomyrdin NV, Katyba GM, Gavdush AA et al. The progress and perspectives of terahertz technology for diagnosis of neoplasms: a review. J Opt 22, 013001 (2020). doi: 10.1088/2040-8986/ab4dc3 [11] Nikitkina AI, Bikmulina PY, Gafarova ER, Kosheleva NV, Efremov YM et al. Terahertz radiation and the skin: a review. J Biomed Opt 26, 043005 (2021). [12] Lindley-Hatcher H, Stantchev RI, Chen X, Hernandez-Serrano AI, Hardwicke J et al. Real time THz imaging—opportunities and challenges for skin cancer detection. Appl Phys Lett 118, 230501 (2021). doi: 10.1063/5.0055259 [13] Reid CB, Fitzgerald A, Reese G, Goldin R, Tekkis P et al. Terahertz pulsed imaging of freshly excised human colonic tissues. Phys Med Biol 56, 4333–4353 (2011). doi: 10.1088/0031-9155/56/14/008 [14] Fitzgerald AJ, Wallace VP, Pinder SE, Purushotham AD, O’Kelly P et al. Classification of terahertz-pulsed imaging data from excised breast tissue. J Biomed Opt 17, 016005 (2012). doi: 10.1117/1.JBO.17.1.016005 [15] Ji YB, Park CH, Kim H, Kim SH, Lee GM et al. Feasibility of terahertz reflectometry for discrimination of human early gastric cancers. Biomed Opt Express 6, 1398–1406 (2015). doi: 10.1364/BOE.6.001398 [16] Chen H, Ma SH, Yan WX, Wu XM, Wang XZ. The diagnosis of human liver cancer by using THz fiber-scanning near-field imaging. Chin Phys Lett 30, 030702 (2013). doi: 10.1088/0256-307X/30/3/030702 [17] Smolyanskaya OA, Chernomyrdin NV, Konovko AA, Zaytsev KI, Ozheredov IA et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog Quantum Electron 62, 1–77 (2018). doi: 10.1016/j.pquantelec.2018.10.001 [18] Shi CJ, Wu X, Peng Y. Applications of terahertz imaging technology in tumor detection. Opto-Electronic Eng 47, 190638 (2020). [19] Joseph CS, Patel R, Neel VA, Giles RH, Yaroslavsky AN. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions optical and terahertz skin cancers imaging. J Biophotonics 7, 295–303 (2014). doi: 10.1002/jbio.201200111 [20] Yaroslavsky A, Joseph C, Patel R, Muzikansky A, Neel VA et al. Delineating nonmelanoma skin cancer margins using terahertz and optical imaging. J Biomed Photonics Eng 3, 010301 (2017). doi: 10.18287/JBPE17.03.010301 [21] Pickwell E, Fitzgerald AJ, Cole BE, Taday PF, Pye RJ et al. Simulating the response of terahertz radiation to basal cell carcinoma using ex vivo spectroscopy measurements. J Biomed Opt 10, 064021 (2005). doi: 10.1117/1.2137667 [22] Pickwell E, Cole BE, Fitzgerald AJ, Pepper M, Wallace VP. In vivo study of human skin using pulsed terahertz radiation. Phys Med Biol 49, 1595–1607 (2004). doi: 10.1088/0031-9155/49/9/001 [23] Zaytsev KI, Gavdush AA, Chernomyrdin NV, Yurchenko SO. Highly accurate in vivo terahertz spectroscopy of healthy skin: variation of refractive index and absorption coefficient along the human body. IEEE Trans Terahertz Sci Technol 5, 817–827 (2015). doi: 10.1109/TTHZ.2015.2460677 [24] Zaitsev KI, Chernomyrdin NV, Kudrin KG, Reshetov IV, Yurchenko SO. Terahertz spectroscopy of pigmentary skin nevi in vivo. Opt Spectrosc 119, 404–410 (2015). doi: 10.1134/S0030400X1509026X [25] Zaytsev KI, Kudrin KG, Karasik VE, Reshetov IV, Yurchenko SO. In vivo terahertz spectroscopy of pigmentary skin nevi: pilot study of non-invasive early diagnosis of dysplasia. Appl Phys Lett 106, 053702 (2015). doi: 10.1063/1.4907350 [26] Sim YC, Park JY, Ahn KM, Park C, Son JH. Terahertz imaging of excised oral cancer at frozen temperature. Biomed Opt Express 4, 1413–1421 (2013). doi: 10.1364/BOE.4.001413 [27] Hernandez-Cardoso GG, Amador-Medina LF, Gutierrez-Torres G, Reyes-Reyes ES, Benavides Martínez CA et al. Terahertz imaging demonstrates its diagnostic potential and reveals a relationship between cutaneous dehydration and neuropathy for diabetic foot syndrome patients. Sci Rep 12, 3110 (2022). doi: 10.1038/s41598-022-06996-w [28] Fan ST, Ung BSY, Parrott EPJ, Wallace VP, Pickwell-MacPherson E. In vivo terahertz reflection imaging of human scars during and after the healing process. J Biophotonics 10, 1143–1151 (2017). doi: 10.1002/jbio.201600171 [29] Musina GR, Chernomyrdin NV, Gafarova ER, Gavdush AA, Shpichka AJ et al. Moisture adsorption by decellularized bovine pericardium collagen matrices studied by terahertz pulsed spectroscopy and solid immersion microscopy. Biomed Opt Express 12, 5368–5386 (2021). doi: 10.1364/BOE.433216 [30] Bajwa N, Au J, Jarrahy R, Sung S, Fishbein MC et al. Non-invasive terahertz imaging of tissue water content for flap viability assessment. Biomed Opt Express 8, 460–474 (2017). doi: 10.1364/BOE.8.000460 [31] Cherkasova OP, Serdyukov DS, Nemova EF, Ratushnyak AS, Kucheryavenko AS et al. Cellular effects of terahertz waves. J Biomed Opt 26, 090902 (2021). [32] DiGirolamo M, Owens JL. Water content of rat adipose tissue and isolated adipocytes in relation to cell size. Am J Physiol 231, 1568–1572 (1976). doi: 10.1152/ajplegacy.1976.231.5.1568 [33] Ashworth PC, Pickwell-MacPherson E, Provenzano E, Pinder SE, Purushotham AD et al. Terahertz pulsed spectroscopy of freshly excised human breast cancer. Opt Express 17, 12444–12454 (2009). doi: 10.1364/OE.17.012444 [34] Sy S, Huang SY, Wang YXJ, Yu J, Ahuja AT et al. Terahertz spectroscopy of liver cirrhosis: investigating the origin of contrast. Phys Med Biol 55, 7587–7596 (2010). doi: 10.1088/0031-9155/55/24/013 [35] Oh SJ, Kim SH, Ji YB, Jeong K, Park Y et al. Study of freshly excised brain tissues using terahertz imaging. Biomed Opt Express 5, 2837–2842 (2014). doi: 10.1364/BOE.5.002837 [36] Joseph CS, Yaroslavsky AN, Al-Arashi M, Goyette TM, Dickinson JC et al. Terahertz spectroscopy of intrinsic biomarkers for non-melanoma skin cancer. Proc SPIE 7215, 72150I (2009). doi: 10.1117/12.809402 [37] Ney M, Abdulhalim I. Comprehensive Monte-Carlo simulator for optimization of imaging parameters for high sensitivity detection of skin cancer at the THz. Proc SPIE 9721, 97210W (2016). [38] Musina GR, Nikitin PV, Chernomyrdin NV, Dolganova IN, Gavdush AA et al. Prospects of terahertz technology in diagnosis of human brain tumors – a review. J Biomed Photonics Eng 6, 020201 (2020). doi: 10.18287/JBPE20.06.020201 [39] Cherkasova O, Peng Y, Konnikova M, Kistenev Y, Shi CJ et al. Diagnosis of glioma molecular markers by terahertz technologies. Photonics 8, 22 (2021). doi: 10.3390/photonics8010022 [40] Chernomyrdin NV, Kucheryavenko AS, Kolontaeva GS, Katyba GM, Dolganova IN et al. Reflection-mode continuous-wave 0.15λ-resolution terahertz solid immersion microscopy of soft biological tissues. Appl Phys Lett 113, 111102 (2018). doi: 10.1063/1.5045480 [41] Chernomyrdin NV, Skorobogatiy M, Gavdush AA, Musina GR, Katyba GM et al. Quantitative super-resolution solid immersion microscopy via refractive index profile reconstruction. Optica 8, 1471–1480 (2021). doi: 10.1364/OPTICA.439286 [42] Cole KS, Cole RH. Dispersion and absorption in dielectrics I. Alternating current characteristics. J Chem Phys 9, 341–351 (1941). doi: 10.1063/1.1750906 [43] Cole KS, Cole RH. Dispersion and absorption in dielectrics II. Direct current characteristics. J Chem Phys 10, 98–105 (1942). doi: 10.1063/1.1723677 [44] Davidson DW. Dielectric relaxation in liquids: I. The representation of relaxation behavior. Can J Chem 39, 571–594 (1961). doi: 10.1139/v61-069 [45] Havriliak S, Negami S. A complex plane analysis of α-dispersions in some polymer systems. J Polym Sci Part C Polym Symp 14, 99–117 (1966). [46] Pickwell E, Cole BE, Fitzgerald AJ, Wallace VP, Pepper M. Simulation of terahertz pulse propagation in biological systems. Appl Phys Lett 84, 2190–2192 (2004). doi: 10.1063/1.1688448 [47] Wang YF, Wang YY, Xu DG, Wu LM, Wang GQ et al. Interference elimination based on the inversion method for continuous-wave terahertz reflection imaging. Opt Express 28, 21926–21939 (2020). doi: 10.1364/OE.396611 [48] Hu BB, Nuss MC. Imaging with terahertz waves. Opt Lett 20, 1716–1718 (1995). doi: 10.1364/OL.20.001716 [49] Gregory IS, Tribe WR, Baker C, Cole BE, Evans MJ et al. Continuous-wave terahertz system with a 60 dB dynamic range. Appl Phys Lett 86, 204104 (2005). doi: 10.1063/1.1935032 [50] Yang X, Zhao X, Yang K, Liu YP, Liu Y et al. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol 34, 810–824 (2016). doi: 10.1016/j.tibtech.2016.04.008 [51] Stoik CD, Bohn MJ, Blackshire JL. Nondestructive evaluation of aircraft composites using transmissive terahertz time domain spectroscopy. Opt Express 16, 17039–17051 (2008). doi: 10.1364/OE.16.017039 [52] Jacobsen RH, Mittleman DM, Nuss MC. Chemical recognition of gases and gas mixtures with terahertz waves. Opt Lett 21, 2011–2013 (1996). doi: 10.1364/OL.21.002011 [53] Cherkasova OP, Nazarov MM, Konnikova M, Shkurinov AP. THz spectroscopy of bound water in glucose: direct measurements from crystalline to dissolved state. J Infrared Millim Terahertz Waves 41, 1057–1068 (2020). doi: 10.1007/s10762-020-00684-4 [54] Komandin GA, Zaytsev KI, Dolganova IN, Nozdrin VS, Chuchupal SV et al. Quantification of solid-phase chemical reactions using the temperature-dependent terahertz pulsed spectroscopy, sum rule, and Arrhenius theory: thermal decomposition of α-lactose monohydrate. Opt Express 30, 9208–9221 (2022). doi: 10.1364/OE.453528 [55] Giuliano BM, Gavdush AA, Müller B, Zaytsev KI, Grassi T et al. Broadband spectroscopy of astrophysical ice analogues. Astron Astrophys 629, A112 (2019). doi: 10.1051/0004-6361/201935619 [56] Lucas J, Géron E, Ditchi T, Holé S. A fast fourier transform implementation of the kramers-kronig relations: application to anomalous and left handed propagation. AIP Adv 2, 032144 (2012). doi: 10.1063/1.4747813 [57] Tuchin VV, Popp J, Zakharov V. Multimodal Optical Diagnostics of Cancer (Springer, Cham, 2020). [58] Lee K, Jeoung K, Kim SH, Ji YB, Son H et al. Measuring water contents in animal organ tissues using terahertz spectroscopic imaging. Biomed Opt Express 9, 1582–1589 (2018). doi: 10.1364/BOE.9.001582 [59] Png GM, Flook R, Ng BWH, Abbott D. Terahertz spectroscopy of snap-frozen human brain tissue: an initial study. Electron Lett 45, 343–345 (2009). doi: 10.1049/el.2009.3413 [60] Meng K, Chen TN, Chen T, Zhu LG, Liu Q et al. Terahertz pulsed spectroscopy of paraffin-embedded brain glioma. J Biomed Opt 19, 077001 (2014). doi: 10.1117/1.JBO.19.7.077001 [61] Shi LY, Shumyatsky P, Rodríguez-Contreras A, Alfano RR. Terahertz spectroscopy of brain tissue from a mouse model of Alzheimer’s disease. J Biomed Opt 21, 015014 (2016). doi: 10.1117/1.JBO.21.1.015014 [62] Tang C, Yang J, Wang YD, Cheng J, Li XL et al. Integrating terahertz metamaterial and water nanodroplets for ultrasensitive detection of amyloid β aggregates in liquids. Sens Actuators B Chem 329, 129113 (2021). doi: 10.1016/j.snb.2020.129113 [63] Musina GR, Dolganova IN, Chernomyrdin NV, Gavdush AA, Ulitko VE et al. Optimal hyperosmotic agents for tissue immersion optical clearing in terahertz biophotonics. J Biophotonics 13, e202000297 (2020). [64] Gavdush AA, Chernomyrdin NV, Malakhov KM, Beshplav SIT, Dolganova IN et al. Terahertz spectroscopy of gelatin-embedded human brain gliomas of different grades: a road toward intraoperative THz diagnosis. J Biomed Opt 24, 027001 (2019). doi: 10.1117/1.JBO.24.2.027001 [65] Gavdush AA, Chernomyrdin NV, Komandin GA, Dolganova IN, Nikitin PV et al. Terahertz dielectric spectroscopy of human brain gliomas and intact tissues ex vivo: double-Debye and double-overdamped-oscillator models of dielectric response. Biomed Opt Express 12, 69–83 (2021). doi: 10.1364/BOE.411025 [66] Yamaguchi S, Fukushi Y, Kubota O, Itsuji T, Ouchi T et al. Brain tumor imaging of rat fresh tissue using terahertz spectroscopy. Sci Rep 6, 30124 (2016). doi: 10.1038/srep30124 [67] Yamaguchi S, Fukushi Y, Kubota O, Itsuji T, Ouchi T et al. Origin and quantification of differences between normal and tumor tissues observed by terahertz spectroscopy. Phys Med Biol 61, 6808–6820 (2016). doi: 10.1088/0031-9155/61/18/6808 [68] Zou Y, Li J, Cui YY, Tang PR, Du LH et al. Terahertz spectroscopic diagnosis of myelin deficit brain in mice and rhesus monkey with chemometric techniques. Sci Rep 7, 5176 (2017). doi: 10.1038/s41598-017-05554-z [69] Mu N, Yang CY, Xu DG, Wang S, Ma K et al. Molecular pathological recognition of freshly excised human glioma using terahertz ATR spectroscopy. Biomed Opt Express 13, 222–236 (2022). doi: 10.1364/BOE.445111 [70] Shiraga K, Ogawa Y, Suzuki T, Kondo N, Irisawa A et al. Determination of the complex dielectric constant of an epithelial cell monolayer in the terahertz region. Appl Phys Lett 102, 053702 (2013). doi: 10.1063/1.4790392 [71] Komandin GA, Anzin VB, Ulitko VE, Gavdush AA, Mukhin AA et al. Optical cryostat with sample rotating unit for polarization-sensitive terahertz and infrared spectroscopy. Opt Eng 59, 061603 (2019). [72] Nozdrin VS, Komandin GA, Spektor IE, Chernomyrdin NV, Seregin DS et al. Optical characteristics of LaNiO3 thin films in the terahertz–infrared frequency range. J Appl Phys 131, 025305 (2022). doi: 10.1063/5.0073466 [73] Komandin GA, Nozdrin VS, Chernomyrdin NV, Seregin DS, Vishnevskiy AS et al. Dielectric permittivity of organosilicate glass thin films on a sapphire substrate determined using time-domain THz and Fourier IR spectroscopy. J Phys D Appl Phys 55, 025303 (2022). doi: 10.1088/1361-6463/ac2ad5 [74] Komandin GA, Chuchupal SV, Lebedev SP, Goncharov YG, Korolev AF et al. BWO generators for terahertz dielectric measurements. IEEE Trans Terahertz Sci Technol 3, 440–444 (2013). doi: 10.1109/TTHZ.2013.2255914 [75] Safian R, Ghazi G, Mohammadian N. Review of photomixing continuous-wave terahertz systems and current application trends in terahertz domain. Opt Eng 58, 110901 (2019). [76] Mine S, Kawase K, Murate K. Real-time wide dynamic range spectrometer using a rapidly wavelength-switchable terahertz parametric source. Opt Lett 46, 2618–2621 (2021). doi: 10.1364/OL.423985 [77] Hafez HA, Kovalev S, Tielrooij KJ, Bonn M, Gensch M et al. Terahertz nonlinear optics of graphene: from saturable absorption to high-harmonics generation. Adv Opt Mater 8, 1900771 (2020). doi: 10.1002/adom.201900771 [78] Burdanova MG, Tsapenko AP, Kharlamova MV, Kauppinen EI, Gorshunov BP et al. A review of the terahertz conductivity and photoconductivity of carbon nanotubes and heteronanotubes. Adv Opt Mater 9, 2101042 (2021). doi: 10.1002/adom.202101042 [79] Dolganova IN, Zaytsev KI, Yurchenko SO, Karasik VE, Tuchin VV. The role of scattering in quasi-ordered structures for terahertz imaging: local order can increase an image quality. IEEE Trans Terahertz Sci Technol 8, 403–409 (2018). doi: 10.1109/TTHZ.2018.2844104 [80] Pickwell-MacPherson E, Wallace VP. Terahertz pulsed imaging—a potential medical imaging modality. Photodiagnosis Photodyn Ther 6, 128–134 (2009). doi: 10.1016/j.pdpdt.2009.07.002 [81] Darmo J, Tamosiunas V, Fasching G, Kröll J, Unterrainer K et al. Imaging with a terahertz quantum cascade laser. Opt Express 12, 1879–1884 (2004). doi: 10.1364/OPEX.12.001879 [82] Kim SM, Hatami F, Harris JS, Kurian AW, Ford J et al. Biomedical terahertz imaging with a quantum cascade laser. Appl Phys Lett 88, 153903 (2006). doi: 10.1063/1.2194229 [83] Zhao HL, Wang YY, Chen LY, Shi J, Ma K et al. High-sensitivity terahertz imaging of traumatic brain injury in a rat model. J Biomed Opt 23, 036015 (2018). doi: 10.1117/1.JBO.23.3.036015 [84] Shi J, Wang YY, Chen TN, Xu DG, Zhao HL et al. Automatic evaluation of traumatic brain injury based on terahertz imaging with machine learning. Opt Express 26, 6371–6381 (2018). doi: 10.1364/OE.26.006371 [85] Ji YB, Oh SJ, Kang SG, Heo J, Kim SH et al. Terahertz reflectometry imaging for low and high grade gliomas. Sci Rep 6, 36040 (2016). doi: 10.1038/srep36040 [86] Wu LM, Xu DG, Wang YY, Liao B, Jiang ZN et al. Study of in vivo brain glioma in a mouse model using continuous-wave terahertz reflection imaging. Biomed Opt Express 10, 3953–3962 (2019). doi: 10.1364/BOE.10.003953 [87] Wang YY, Sun ZC, Xu DG, Wu LM, Chang JY et al. A hybrid method based region of interest segmentation for continuous wave terahertz imaging. J Phys D Appl Phys 53, 095403 (2020). doi: 10.1088/1361-6463/ab58b6 [88] Wu LM, Xu DG, Wang YY, Zhang YY, Wang HJ et al. Horizontal-scanning attenuated total reflection terahertz imaging for biological tissues. Neurophotonics 7, 025005 (2020). [89] Wu LM, Wang YY, Liao B, Zhao L, Chen K et al. Temperature dependent terahertz spectroscopy and imaging of orthotopic brain gliomas in mouse models. Biomed Opt Express 13, 93–104 (2022). doi: 10.1364/BOE.445597 [90] Wu LM, Xu DG, Wang YY, Ge ML, Li HB et al. Common path continuous terahertz reflection and attenuated total reflection imaging. Acta Phys Sin 70, 118701 (2021). doi: 10.7498/aps.70.20210182 [91] Dolganova IN, Zaytsev KI, Metelkina AA, Karasik VE, Yurchenko SO. A hybrid continuous-wave terahertz imaging system. Rev Sci Instrum 86, 113704 (2015). doi: 10.1063/1.4935495 [92] Lo YH, Leonhardt R. Aspheric lenses for terahertz imaging. Opt Express 16, 15991–15998 (2008). doi: 10.1364/OE.16.015991 [93] Chernomyrdin NV, Frolov ME, Lebedev SP, Reshetov IV, Spektor IE et al. Wide-aperture aspherical lens for high-resolution terahertz imaging. Rev Sci Instrum 88, 014703 (2017). doi: 10.1063/1.4973764 [94] Chernomyrdin NV, Skorobogatiy M, Ponomarev DS, Bukin VV, Tuchin VV et al. Terahertz solid immersion microscopy: recent achievements and challenges. Appl Phys Lett 120, 110501 (2022). doi: 10.1063/5.0085906 [95] Ahi K. Mathematical modeling of THz point spread function and simulation of THz imaging systems. IEEE Trans Terahertz Sci Technol 7, 747–754 (2017). doi: 10.1109/TTHZ.2017.2750690 [96] Wang Y, Qi F, Wang JK. Terahertz image super-resolution based on a complex convolutional neural network. Opt Lett 46, 3123–3126 (2021). doi: 10.1364/OL.422684 [97] McClatchey K, Reiten MT, Cheville RA. Time resolved synthetic aperture terahertz impulse imaging. Appl Phys Lett 79, 4485–4487 (2001). doi: 10.1063/1.1427745 [98] Guerboukha H, Nallappan K, Skorobogatiy M. Exploiting k-space/frequency duality toward real-time terahertz imaging. Optica 5, 109–116 (2018). doi: 10.1364/OPTICA.5.000109 [99] Petrov NV, Perraud JB, Chopard A, Guillet JP, Smolyanskaya OA et al. Terahertz phase retrieval imaging in reflection. Opt Lett 45, 4168–4171 (2020). doi: 10.1364/OL.397935 [100] Heimbeck MS, Everitt HO. Terahertz digital holographic imaging. Adv Opt Photonics 12, 1–59 (2020). doi: 10.1364/AOP.12.000001 [101] Zanotto L, Piccoli R, Dong JL, Morandotti R, Razzari L. Single-pixel terahertz imaging: a review. Opto-Electron Adv 3, 200012 (2020). [102] van der Valk NCJ, Planken PCM. Electro-optic detection of subwavelength terahertz spot sizes in the near field of a metal tip. Appl Phys Lett 81, 1558–1560 (2002). doi: 10.1063/1.1503404 [103] Chen HT, Kersting R, Cho GC. Terahertz imaging with nanometer resolution. Appl Phys Lett 83, 3009–3011 (2003). doi: 10.1063/1.1616668 [104] Huber AJ, Keilmann F, Wittborn J, Aizpurua J, Hillenbrand R. Terahertz near-field nanoscopy of mobile carriers in single semiconductor nanodevices. Nano Lett 8, 3766–3770 (2008). doi: 10.1021/nl802086x [105] Buron JD, Petersen DH, Bøggild P, Cooke DG, Hilke M et al. Graphene conductance uniformity mapping. Nano Lett 12, 5074–5081 (2012). doi: 10.1021/nl301551a [106] Simovski CR, Belov PA, Atrashchenko AV, Kivshar YS. Wire metamaterials: physics and applications. Adv Mater 24, 4229–4248 (2012). doi: 10.1002/adma.201200931 [107] Habib MS, Stefani A, Atakaramians S, Fleming SC, Argyros A et al. A prism based magnifying hyperlens with broad-band imaging. Appl Phys Lett 110, 101106 (2017). doi: 10.1063/1.4978445 [108] Schade U, Holldack K, Kuske P, Wüstefeld G, Hübers HW. THz near-field imaging employing synchrotron radiation. Appl Phys Lett 84, 1422–1424 (2004). doi: 10.1063/1.1650034 [109] Ishihara K, Ohashi K, Ikari T, Minamide H, Yokoyama H et al. Terahertz-wave near-field imaging with subwavelength resolution using surface-wave-assisted bow-tie aperture. Appl Phys Lett 89, 201120 (2006). doi: 10.1063/1.2387984 [110] Macfaden AJ, Reno JL, Brener I, Mitrofanov O. 3 μm aperture probes for near-field terahertz transmission microscopy. Appl Phys Lett 104, 011110 (2014). doi: 10.1063/1.4861621 [111] Stantchev RI, Sun BQ, Hornett SM, Hobson PA, Gibson GM et al. Noninvasive, near-field terahertz imaging of hidden objects using a single-pixel detector. Sci Adv 2, e1600190 (2016). doi: 10.1126/sciadv.1600190 [112] Okada K, Serita K, Zang ZR, Murakami H, Kawayama I et al. Scanning laser terahertz near-field reflection imaging system. Appl Phys Express 12, 122005 (2019). doi: 10.7567/1882-0786/ab4ddf [113] Chernomyrdin NV, Schadko AO, Lebedev SP, Tolstoguzov VL, Kurlov VN et al. Solid immersion terahertz imaging with sub-wavelength resolution. Appl Phys Lett 110, 221109 (2017). doi: 10.1063/1.4984952 [114] Chernomyrdin NV, Zhelnov VA, Kucheryavenko AS, Dolganova IN, Katyba GM et al. Numerical analysis and experimental study of terahertz solid immersion microscopy. Opt Eng 59, 061605 (2019). [115] Zhelnov VA, Zaytsev KI, Kucheryavenko AS, Katyba GM, Dolganova IN et al. Object-dependent spatial resolution of the reflection-mode terahertz solid immersion microscopy. Opt Express 29, 3553–3566 (2021). doi: 10.1364/OE.415049 [116] Kucheryavenko AS, Chernomyrdin NV, Gavdush AA, Alekseeva AI, Nikitin PV et al. Terahertz dielectric spectroscopy and solid immersion microscopy of ex vivo glioma model 101.8: brain tissue heterogeneity. Biomed Opt Express 12, 5272–5289 (2021). doi: 10.1364/BOE.432758 [117] Wang ZB, Guo W, Li L, Luk’yanchuk B, Khan A et al. Optical virtual imaging at 50 nm lateral resolution with a white-light nanoscope. Nat Commun 2, 218 (2011). doi: 10.1038/ncomms1211 [118] Upputuri PK, Pramanik M. Microsphere-aided optical microscopy and its applications for super-resolution imaging. Opt Commun 404, 32–41 (2017). doi: 10.1016/j.optcom.2017.05.049 [119] Chen LW, Zhou Y, Li Y, Hong MH. Microsphere enhanced optical imaging and patterning: from physics to applications. Appl Phys Rev 6, 021304 (2019). doi: 10.1063/1.5082215 [120] Chen XX, Wu TL, Gong ZY, Li YC, Zhang Y et al. Subwavelength imaging and detection using adjustable and movable droplet microlenses. Photonics Res 8, 03000225 (2020). [121] Perrin S, Donie YJ, Montgomery P, Gomard G, Lecler S. Compensated microsphere-assisted interference microscopy. Phys Rev Appl 13, 014068 (2020). doi: 10.1103/PhysRevApplied.13.014068 [122] Chen LW, Zhou Y, Wu MX, Hong MH. Remote-mode microsphere nano-imaging: new boundaries for optical microscopes. Opto-Electron Adv 1, 170001 (2018). [123] Zhu YC, Chen XL, Yuan WZ, Chu ZQ, Wong KY et al. A waveguide metasurface based quasi-far-field transverse-electric superlens. Opto-Electron Adv 4, 210013 (2021). doi: 10.29026/oea.2021.210013 [124] Geng GS, Dai GB, Li DD, Zhou SL, Li ZX et al. Imaging brain tissue slices with terahertz near-field microscopy. Biotechnol Prog 35, e2741 (2019). doi: 10.1002/btpr.2741 [125] Watanabe T, Wang XQ, Tan ZG, Frahm J. Magnetic resonance imaging of brain cell water. Sci Rep 9, 5084 (2019). doi: 10.1038/s41598-019-41587-2 [126] Liu GZ, Chang C, Qiao Z, Wu KJ, Zhu Z et al. Myelin sheath as a dielectric waveguide for signal propagation in the mid-infrared to terahertz spectral range. Adv Funct Mater 29, 1807862 (2019). doi: 10.1002/adfm.201807862 [127] Herbert E, Engel-Hills P, Hattingh C, Fouche JP, Kidd M et al. Fractional anisotropy of white matter, disability and blood iron parameters in multiple sclerosis. Metab Brain Dis 33, 545–557 (2018). doi: 10.1007/s11011-017-0171-5 [128] Dong DB, Wang YL, Chang XB, Chen X, Chang X et al. Common and diagnosis-specific fractional anisotropy of white matter in schizophrenia, bipolar disorder, and major depressive disorder: evidence from comparative voxel-based meta-analysis. Schizophr Res 193, 456–458 (2018). doi: 10.1016/j.schres.2017.07.003 [129] Owens JA, Spitz G, Ponsford JL, Dymowski AR, Willmott C. An investigation of white matter integrity and attention deficits following traumatic brain injury. Brain Inj 32, 776–783 (2018). doi: 10.1080/02699052.2018.1451656 [130] Wang J, Xu SL, Duan JJ, Yi L, Guo YF et al. Invasion of white matter tracts by glioma stem cells is regulated by a NOTCH1–SOX2 positive-feedback loop. Nat Neurosci 22, 91–105 (2019). doi: 10.1038/s41593-018-0285-z [131] Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J et al. Dementia prevention, intervention, and care. Lancet 390, 2673–2734 (2017). doi: 10.1016/S0140-6736(17)31363-6 [132] Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med 362, 329–344 (2010). doi: 10.1056/NEJMra0909142 [133] Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608 (2016). doi: 10.15252/emmm.201606210 [134] Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease — insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13, 612–623 (2017). doi: 10.1038/nrneurol.2017.111 [135] Laske C, Sohrabi HR, Frost SM, López-de-Ipiña K, Garrard P et al. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimers Dement 11, 561–578 (2015). doi: 10.1016/j.jalz.2014.06.004 [136] Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer’s disease. Nat Med 10, S34–S41 (2004). doi: 10.1038/nrn1433 [137] Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006). doi: 10.1038/nature05291 [138] Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24, 1583 (2019). doi: 10.3390/molecules24081583 [139] Pereira TMC, Côco LZ, Ton AMM, Meyrelles SS, Campos-Toimil M et al. The emerging scenario of the gut–brain axis: the therapeutic actions of the new actor kefir against neurodegenerative diseases. Antioxidants 10, 1845 (2021). doi: 10.3390/antiox10111845 [140] Lee SH, Shin S, Roh Y, Oh SJ, Lee SH et al. Label-free brain tissue imaging using large-area terahertz metamaterials. Biosens Bioelectron 170, 112663 (2020). doi: 10.1016/j.bios.2020.112663 [141] Heo C, Ha T, You C, Huynh T, Lim H et al. Identifying fibrillization state of Aβ protein via near-field THz conductance measurement. ACS Nano 14, 6548–6558 (2020). doi: 10.1021/acsnano.9b08572 [142] Yeo WG, Gurel O, Srinivasan N, King PD, Nahar NK et al. Terahertz imaging and electromagnetic model of axon demyelination in Alzheimer’s disease. IEEE Trans Terahertz Sci Technol 7, 711–721 (2017). doi: 10.1109/TTHZ.2017.2739481 [143] Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21, v1–v100 (2019). doi: 10.1093/neuonc/noz150 [144] Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol 44, 139–150 (2018). doi: 10.1111/nan.12432 [145] Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2, 1460–1469 (2016). doi: 10.1001/jamaoncol.2016.1373 [146] Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131, 803–820 (2016). doi: 10.1007/s00401-016-1545-1 [147] Rasmussen IA Jr, Lindseth F, Rygh OM, Berntsen EM, Selbekk T et al. Functional neuronavigation combined with intra-operative 3D ultrasound: Initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien) 149, 365–378 (2007). doi: 10.1007/s00701-006-1110-0 [148] Senft C, Bink A, Franz K, Vatter H, Gasser T et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12, 997–1003 (2011). doi: 10.1016/S1470-2045(11)70196-6 [149] Pustogarov N, Panteleev D, Goryaynov SA, Ryabova AV, Rybalkina EY et al. Hiding in the shadows: CPOX expression and 5-ALA induced fluorescence in human glioma cells. Mol Neurobiol 54, 5699–5708 (2017). doi: 10.1007/s12035-016-0109-7 [150] Chen B, Wang HF, Ge PF, Zhao JW, Li WC et al. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci 9, 708–714 (2012). doi: 10.7150/ijms.4843 [151] Goryaynov SA, Okhlopkov VA, Golbin DA, Chernyshov KA, Svistov DV et al. Fluorescence diagnosis in neurooncology: retrospective analysis of 653 cases. Front Oncol 9, 830 (2019). doi: 10.3389/fonc.2019.00830 [152] Kiseleva EB, Yashin KS, Moiseev AA, Timofeeva LB, Kudelkina VV et al. Optical coefficients as tools for increasing the optical coherence tomography contrast for normal brain visualization and glioblastoma detection. Neurophotonics 6, 035003 (2019). [153] Dolganova IN, Aleksandrova PV, Nikitin PV, Alekseeva AI, Chernomyrdin NV et al. Capability of physically reasonable OCT-based differentiation between intact brain tissues, human brain gliomas of different WHO grades, and glioma model 101.8 from rats. Biomed Opt Express 11, 6780–6798 (2020). doi: 10.1364/BOE.409692 [154] Orringer DA, Pandian B, Niknafs YS, Hollon TC, Boyle J et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat Biomed Eng 1, 0027 (2017). doi: 10.1038/s41551-016-0027 [155] Feng X, Muzikansky A, Ross AH, Hamblin MR, Jermain PR et al. Multimodal quantitative imaging of brain cancer in cultured cells. Biomed Opt Express 10, 4237–4248 (2019). doi: 10.1364/BOE.10.004237 [156] Genina EA, Bashkatov AN, Tuchina DK, Dyachenko PA, Navolokin N et al. Optical properties of brain tissues at the different stages of glioma development in rats: pilot study. Biomed Opt Express 10, 5182–5197 (2019). doi: 10.1364/BOE.10.005182 [157] Kircher MF, De La Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med 18, 829–834 (2012). doi: 10.1038/nm.2721 [158] Fan ST, Ung B, Parrott EPJ, Pickwell-MacPherson E. Gelatin embedding: a novel way to preserve biological samples for terahertz imaging and spectroscopy. Phys Med Biol 60, 2703–2713 (2015). doi: 10.1088/0031-9155/60/7/2703 [159] Komandin GA, Nozdrin VS, Gavdush AA, Pronin AA, Porodinkov OE et al. Effect of moisture adsorption on the broadband dielectric response of SiO2-based nanoporous glass. J Appl Phys 126, 224303 (2019). doi: 10.1063/1.5116790 [160] McIntyre GI. Cell hydration as the primary factor in carcinogenesis: a unifying concept. Med Hypotheses 66, 518–526 (2006). doi: 10.1016/j.mehy.2005.09.022 [161] Truong BCQ, Tuan HD, Wallace VP, Fitzgerald AJ, Nguyen HT. The potential of the double debye parameters to discriminate between basal cell carcinoma and normal skin. IEEE Trans Terahertz Sci Technol 5, 990–998 (2015). doi: 10.1109/TTHZ.2015.2485208 [162] Abbas Z, Gras V, Möllenhoff K, Oros-Peusquens AM, Shah NJ. Quantitative water content mapping at clinically relevant field strengths: a comparative study at 1.5T and 3T. NeuroImage 106, 404–413 (2015). doi: 10.1016/j.neuroimage.2014.11.017 [163] Neeb H, Zilles K, Shah NJ. A new method for fast quantitative mapping of absolute water content in vivo. NeuroImage 31, 1156–1168 (2006). doi: 10.1016/j.neuroimage.2005.12.063 [164] Neeb H, Ermer V, Stocker T, Shah NJ. Fast quantitative mapping of absolute water content with full brain coverage. NeuroImage 42, 1094–1109 (2008). doi: 10.1016/j.neuroimage.2008.03.060 [165] DiResta GR, Lee J, Arbit E. Measurement of brain tissue specific gravity using pycnometry. J Neurosci Methods 39, 245–251 (1991). doi: 10.1016/0165-0270(91)90103-7 [166] Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta 491, 39–45 (2019). doi: 10.1016/j.cca.2019.01.011 [167] Sobecki M, Mrouj K, Camasses A, Parisis N, Nicolas E et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 5, e13722 (2016). doi: 10.7554/eLife.13722 [168] Wong E, Nahar N, Hau E, Varikatt W, Gebski V et al. Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac J Clin Oncol 15, 5–9 (2019). [169] Henker C, Kriesen T, Schneider B, Glass Ä, Scherer M et al. Correlation of Ki-67 index with volumetric segmentation and its value as a prognostic marker in glioblastoma. World Neurosurg 125, e1093–e1103 (2019). doi: 10.1016/j.wneu.2019.02.006 [170] Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, metabolism, and cancer. Cancer Discov 5, 1024–1039 (2015). doi: 10.1158/2159-8290.CD-15-0507 [171] Turkalp Z, Karamchandani J, Das S. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol 71, 1319–1325 (2014). doi: 10.1001/jamaneurol.2014.1205 [172] Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 164, 550–563 (2016). doi: 10.1016/j.cell.2015.12.028 [173] Bell EH, Zhang PX, Fisher BJ, Macdonald DR, McElroy JP et al. Association of MGMT promoter methylation status with survival outcomes in patients with high-risk glioma treated with radiotherapy and temozolomide: an analysis from the NRG oncology/RTOG 0424 trial. JAMA Oncol 4, 1405–1409 (2018). doi: 10.1001/jamaoncol.2018.1977 [174] Sasaki T, Kinoshita M, Fujita K, Fukai J, Hayashi N et al. Radiomics and MGMT promoter methylation for prognostication of newly diagnosed glioblastoma. Sci Rep 9, 14435 (2019). doi: 10.1038/s41598-019-50849-y [175] Chen WQ, Peng Y, Jiang XK, Zhao JY, Zhao HW et al. Isomers identification of 2-hydroxyglutarate acid disodium salt (2HG) by terahertz time-domain spectroscopy. Sci Rep 7, 12166 (2017). doi: 10.1038/s41598-017-11527-z [176] Fedoseeva VV, Postovalova EA, Khalansky AS, Razzhivina VA, Gelperina SE et al. Drug-induced pathomorphosis of glioblastoma 101.8 in wistar rats treated with doxorubicin bound to poly (lactide-co-glycolide) nanoparticles. Sovrem Tekhnologii Med 10, 105 (2018). doi: 10.17691/stm2018.10.4.12 [177] Chernomyrdin NV, Gavdush AA, Beshplav SIT, Malakhov KM, Kucheryavenko AS et al. In vitro terahertz spectroscopy of gelatin-embedded human brain tumors: a pilot study. Proc SPIE 10716, 107160S (2018). [178] Wang YY, Jiang ZN, Xu DG, Chen TN, Chen BK et al. Study of the dielectric characteristics of living glial-like cells using terahertz ATR spectroscopy. Biomed Opt Express 10, 5351–5361 (2019). doi: 10.1364/BOE.10.005351 [179] Liao YS, Zhang MK, Tang MJ, Chen LG, Li XQ et al. Label-free study on the effect of a bioactive constituent on glioma cells in vitro using terahertz ATR spectroscopy. Biomed Opt Express 13, 2380–2392 (2022). doi: 10.1364/BOE.452952 [180] Ghajar J. Traumatic brain injury. Lancet 356, 923–929 (2000). doi: 10.1016/S0140-6736(00)02689-1 [181] Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 6, 393–403 (2010). doi: 10.1038/nrneurol.2010.74 [182] Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6, 137–192 (2012). doi: 10.1007/s11682-012-9156-5 [183] Wunder A, Schoknecht K, Stanimirovic DB, Prager O, Chassidim Y. Imaging blood-brain barrier dysfunction in animal disease models. Epilepsia 53, 14–21 (2012). [184] Coles JP, Fryer TD, Smielewski P, Rice K, Clark JC et al. Defining ischemic burden after traumatic brain injury using 15O PET imaging of cerebral physiology. J Cereb Blood Flow Metab 24, 191–201 (2004). doi: 10.1097/01.WCB.0000100045.07481.DE [185] Yang SH, Xing D, Lao YQ, Yang DW, Zeng LM et al. Noninvasive monitoring of traumatic brain injury and post-traumatic rehabilitation with laser-induced photoacoustic imaging. Appl Phys Lett 90, 243902 (2007). doi: 10.1063/1.2749185 [186] Zhang XD, Wang HS, Antaris AL, Li LL, Diao S et al. Traumatic brain injury imaging in the second near-infrared window with a molecular fluorophore. Adv Mater 28, 6872–6879 (2016). doi: 10.1002/adma.201600706 [187] Wang YY, Wang GQ, Xu DG, Jiang BZ, Ge ML et al. Terahertz spectroscopic diagnosis of early blast-induced traumatic brain injury in rats. Biomed Opt Express 11, 4085–4098 (2020). doi: 10.1364/BOE.395432 [188] Bashkatov AN, Berezin KV, Dvoretskiy KN, Chernavina ML, Genina EA et al. Measurement of tissue optical properties in the context of tissue optical clearing. J Biomed Opt 23, 091416 (2018). [189] Tuchin VV. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics 3rd ed (SPIE Press, Bellingham, Washington, USA, 2015). [190] Ishimaru A. Electromagnetic Wave Propagation, Radiation, and Scattering: From Fundamentals to Applications (Wiley-IEEE Press, Hoboken, New Jersey, USA, 2017). [191] Chernomyrdin NV, Kucheryavenko AS, Rimskaya EN, Dolganova IN, Zhelnov VA et al. Terahertz microscope based on solid immersion effect for imaging of biological tissues. Opt Spectrosc 126, 560–567 (2019). doi: 10.1134/S0030400X19050059 [192] Li ZX, Yan SH, Zang ZY, Geng GS, Yang ZB et al. Single cell imaging with near-field terahertz scanning microscopy. Cell Prolif 53, e12788 (2020). [193] Neftel C, Laffy J, Filbin MG, Hara T, Shore ME et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849.e21 (2019). doi: 10.1016/j.cell.2019.06.024 [194] Dirkse A, Golebiewska A, Buder T, Nazarov PV, Muller A et al. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat Commun 10, 1787 (2019). doi: 10.1038/s41467-019-09853-z [195] Zhou D, Alver BM, Li S, Hlady RA, Thompson JJ et al. Distinctive epigenomes characterize glioma stem cells and their response to differentiation cues. Genome Biol 19, 43 (2018). doi: 10.1186/s13059-018-1420-6 [196] Behnan J, Finocchiaro G, Hanna G. The landscape of the mesenchymal signature in brain tumours. Brain 142, 847–866 (2019). doi: 10.1093/brain/awz044 [197] Ren XW, Kang BX, Zhang ZM. Understanding tumor ecosystems by single-cell sequencing: promises and limitations. Genome Biol 19, 211 (2018). doi: 10.1186/s13059-018-1593-z [198] Guerboukha H, Nallappan K, Cao Y, Seghilani M, Azaña J et al. Planar porous components for low-loss terahertz optics. Adv Opt Mater 7, 1900236 (2019). doi: 10.1002/adom.201900236 [199] Ulitko VE, Zotov AK, Gavdush AA, Katyba GM, Komandin GA et al. Nanoporous SiO2 based on annealed artificial opals as a favorable material platform of terahertz optics. Opt Mater Express 10, 2100–2113 (2020). doi: 10.1364/OME.402185 [200] Ulitko VE, Katyba GM, Zhelnov VA, Shmytko IM, Emelchenko GA et al. Opal-based terahertz optical elements fabricated by self-assembly of porous SiO2 nanoparticles. Opt Express 29, 13764–13777 (2021). doi: 10.1364/OE.422637 [201] Li JT, Wang GC, Yue Z, Liu JY, Li J et al. Dynamic phase assembled terahertz metalens for reversible conversion between linear polarization and arbitrary circular polarization. Opto-Electron Adv 5, 210062 (2022). doi: 10.29026/oea.2022.210062 [202] Katyba GM, Chizhov PA, Kurlov VN, Dolganova IN, Garnov SV et al. THz generation by two-color laser air plasma coupled to antiresonance hollow-core sapphire waveguides: THz-wave delivery and angular distribution management. Opt Express 30, 4215–4230 (2022). doi: 10.1364/OE.447060 [203] Katyba GM, Zaytsev KI, Chernomyrdin NV, Shikunova IA, Komandin GA et al. Sapphire photonic crystal waveguides for terahertz sensing in aggressive environments. Adv Opt Mater 6, 1800573 (2018). doi: 10.1002/adom.201800573 [204] Ponomarev DS, Lavrukhin DV, Zenchenko NV, Frolov TV, Glinskiy IA et al. Boosting photoconductive large-area THz emitter via optical light confinement behind a highly refractive sapphire-fiber lens. Opt Lett 47, 1899–1902 (2022). doi: 10.1364/OL.452192 [205] Lepeshov S, Gorodetsky A, Krasnok A, Rafailov E, Belov P. Enhancement of terahertz photoconductive antenna operation by optical nanoantennas. Laser Photon Rev 11, 1600199 (2017). doi: 10.1002/lpor.201600199 [206] Lavrukhin DV, Yachmenev AE, Glinskiy IA, Khabibullin RA, Goncharov YG et al. Terahertz photoconductive emitter with dielectric-embedded high-aspect-ratio plasmonic grating for operation with low-power optical pumps. AIP Adv 9, 015112 (2019). doi: 10.1063/1.5081119 [207] Henri R, Nallappan K, Ponomarev DS, Guerboukha H, Lavrukhin DV et al. Fabrication and characterization of an 8 × 8 terahertz photoconductive antenna array for spatially resolved time domain spectroscopy and imaging applications. IEEE Access 9, 117691–117702 (2021). doi: 10.1109/ACCESS.2021.3106227 [208] Guerboukha H, Markov A, Qu H, Skorobogatiy M. Time resolved dynamic measurements at THz frequencies using a rotary optical delay line. IEEE Trans Terahertz Sci Technol 5, 564–572 (2015). doi: 10.1109/TTHZ.2015.2441701 [209] Skorobogatiy M. Linear rotary optical delay lines. Opt Express 22, 11812–11833 (2014). doi: 10.1364/OE.22.011812 [210] Ryzhii V, Otsuji T, Shur M. Graphene based plasma-wave devices for terahertz applications. Appl Phys Lett 116, 140501 (2020). doi: 10.1063/1.5140712 [211] Tong MY, Hu YZ, Xie XN, Zhu XG, Wang ZY et al. Helicity-dependent THz emission induced by ultrafast spin photocurrent in nodal-line semimetal candidate Mg3Bi2. Opto-Electron Adv 3, 200023 (2020). [212] Lazareva EN, Oliveira L, Yanina IY, Chernomyrdin NV, Musina GR et al. Refractive index measurements of tissue and blood components and OCAs in a wide spectral range. In Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging (eds. Tuchin VV, Zhu D, Genina EA) 141–166 (CRC Press, Boca Raton, FL, USA, 2022). [213] Smolyanskaya OA, Zaytsev KI, Dolganova IN, Musina GR, Tuchina DK et al. Tissue optical clearing in the terahertz range. In Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging (eds. Tuchin VV, Zhu D, Genina EA) 445–458 (CRC Press, Boca Raton, FL, USA, 2022). [214] Tuchin VV, Zhu D, Genina EA. Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging (CRC Press, Boca Raton, FL USA, 2022). [215] Musina GR, Gavdush AA, Chernomyrdin NV, Dolganova IN, Ulitko VE et al. Optical properties of hyperosmotic agents for immersion clearing of tissues in terahertz spectroscopy. Opt Spectrosc 128, 1026–1035 (2020). doi: 10.1134/S0030400X20070279 [216] Oh SJ, Kim SH, Jeong K, Park Y, Huh YM et al. Measurement depth enhancement in terahertz imaging of biological tissues. Opt Express 21, 21299–21305 (2013). doi: 10.1364/OE.21.021299 [217] Xiao YD, Paudel R, Liu J, Ma C, Zhang ZS et al. MRI contrast agents: classification and application (review). Int J Mol Med 38, 1319–1326 (2016). doi: 10.3892/ijmm.2016.2744 [218] Faucher L, Tremblay M, Lagueux J, Gossuin Y, Fortin MA. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Appl Mater Interfaces 4, 4506–4515 (2012). doi: 10.1021/am3006466 [219] Rutten A, Prokop M. Contrast agents in X-ray computed tomography and its applications in oncology. Anticancer Agents Med Chem 7, 307–316 (2007). doi: 10.2174/187152007780618162 [220] Xu XY, Liu K, Wang Y, Zhang CC, Shi MH et al. A multifunctional low-generation dendrimer-based nanoprobe for the targeted dual mode MR/CT imaging of orthotopic brain gliomas. J Mater Chem B 7, 3639–3643 (2019). doi: 10.1039/C9TB00416E [221] Mahmoudi K, Garvey KL, Bouras A, Cramer G, Stepp H et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neurooncol 141, 595–607 (2019). doi: 10.1007/s11060-019-03103-4 [222] Xu WD, Xie LJ, Zhu JF, Xu X, Ye ZZ et al. Gold nanoparticle-based terahertz metamaterial sensors: mechanisms and applications. ACS Photonics 3, 2308–2314 (2016). doi: 10.1021/acsphotonics.6b00463 [223] RoyChoudhury S, Rawat V, Jalal AH, Kale SN, Bhansali S. Recent advances in metamaterial split-ring-resonator circuits as biosensors and therapeutic agents. Biosens Bioelectron 86, 595–608 (2016). doi: 10.1016/j.bios.2016.07.020 [224] Peng Y, Shi CJ, Wu X, Zhu YM, Zhuang SL. Terahertz imaging and spectroscopy in cancer diagnostics: a technical review. BME Front 2020, 2547609 (2020). [225] Peng Y, Shi CJ, Zhu YM, Gu M, Zhuang SL. Terahertz spectroscopy in biomedical field: a review on signal-to-noise ratio improvement. PhotoniX 1, 12 (2020). doi: 10.1186/s43074-020-00011-z [226] Yang K, Li JN, Lamy de la Chapelle M, Huang GR, Wang YX et al. A terahertz metamaterial biosensor for sensitive detection of microRNAs based on gold-nanoparticles and strand displacement amplification. Biosens Bioelectron 175, 112874 (2021). doi: 10.1016/j.bios.2020.112874 [227] Liu K, Zhang R, Liu Y, Chen XQ, Li KD et al. Gold nanoparticle enhanced detection of EGFR with a terahertz metamaterial biosensor. Biomed Opt Express 12, 1559–1567 (2021). doi: 10.1364/BOE.418859 [228] Yan ZY, Zhu LG, Meng K, Huang WX, Shi QW. THz medical imaging: from in vitro to in vivo. Trends Biotechnol 40, 816–830 (2022). doi: 10.1016/j.tibtech.2021.12.002 [229] Huang QQ, Zou Y, Zhong SC, Yang X, Li J et al. Silica-coated gold nanorods with high photothermal efficiency and biocompatibility as a contrast agent for in vitro terahertz imaging. J Biomed Nanotechnol 15, 910–920 (2019). doi: 10.1166/jbn.2019.2738 [230] Cristian CR, Thomas S, Vasile D, Stanciu GD, Alexa-Stratulat T et al. Research on functionalized gadolinium oxide nanoparticles for MRI and THz imaging. In 2018 International Conference and Exposition on Electrical And Power Engineering (EPE) 646–649 (IEEE, 2018);http://doi.org/10.1109/ICEPE.2018.8559855. [231] Smolyanskaya OA, Lazareva EN, Nalegaev SS, Petrov NV, Zaytsev KI et al. Multimodal optical diagnostics of glycated biological tissues. Biochemistry (Mosc) 84, 124–143 (2019). doi: 10.1134/S0006297919140086 [232] Chernomyrdin NV, Dolganova IN, Beshplav SIT, Aleksandrova PV, Musina GR et al. Differentiation of healthy and malignant brain tissues using terahertz pulsed spectroscopy and optical coherence tomography. Proc SPIE 10864, 1086406 (2019). [233] Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG et al. Optical coherence tomography. Science 254, 1178–1181 (1991). doi: 10.1126/science.1957169 [234] Patel R, Khan A, Quinlan R, Yaroslavsky AN. Polarization-sensitive multimodal imaging for detecting breast cancer. Cancer Res 74, 4685–4693 (2014). [235] Jones BF. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans Med Imaging 17, 1019–1027 (1998). doi: 10.1109/42.746635 [236] Chernomyrdin NV, Lesnichaya AD, Yakovlev EV, Kudrin KG, Cherkasova OP et al. Differentiation of basal cell carcinoma and healthy skin using multispectral modulation autofluorescence imaging: a pilot study. J Biomed Photonics Eng 5, 010302 (2019). doi: 10.18287/JBPE19.05.010302 [237] Stenquist B, Ericson MB, Strandeberg C, Mölne L, Rosén A et al. Bispectral fluorescence imaging of aggressive basal cell carcinoma combined with histopathological mapping: a preliminary study indicating a possible adjunct to Mohs micrographic surgery. Br J Dermatol 154, 305–309 (2006). doi: 10.1111/j.1365-2133.2005.07035.x [238] Kleinerman R, Whang TB, Bard RL, Marmur ES. Ultrasound in dermatology: Principles and applications. J Am Acad Dermatol 67, 478–487 (2012). doi: 10.1016/j.jaad.2011.12.016 [239] Lui H, Zhao JH, McLean D, Zeng HS. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res 72, 2491–2500 (2012). [240] Fan B, Neel VA, Yaroslavsky AN. Multimodal imaging for nonmelanoma skin cancer margin delineation. Lasers Surg Med 49, 319–326 (2017). doi: 10.1002/lsm.22552 [241] Paoli J, Smedh M, Wennberg AM, Ericson MB. Multiphoton laser scanning microscopy on non-melanoma skin cancer: morphologic features for future non-invasive diagnostics. J Invest Dermatol 128, 1248–1255 (2008). doi: 10.1038/sj.jid.5701139 -
Access History
Article Metrics
-
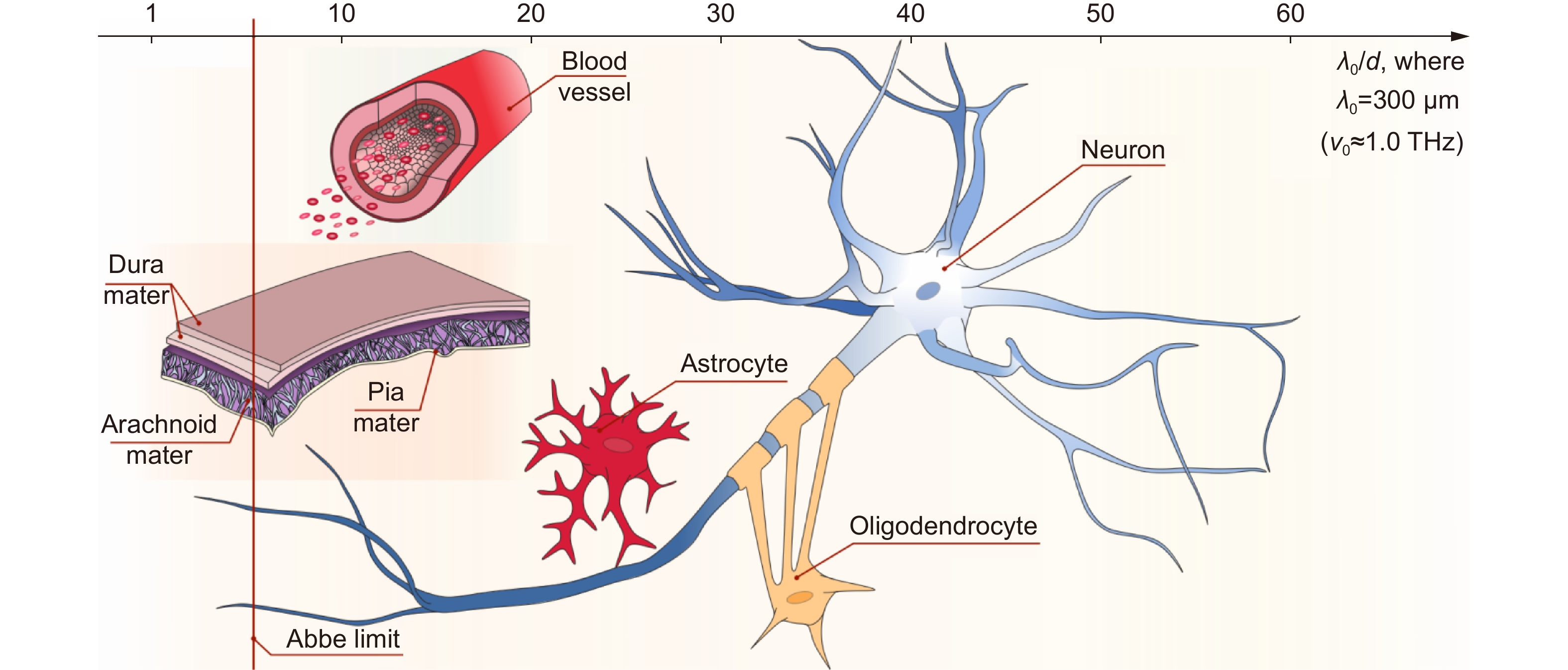
Figure 1.
Structural features of brain tissues (neurofibrils, neurons, glial cells, etc.) as well as meninges and blood vessels at the THz-wavelength scale. The horizontal axis depicts the ratio between the typical size of the structural elements d and the typical free-space wavelength λ0= 300 μm (ν0≈ 1.0 THz), while the vertical solid red line shows the λ/2-Abbe diffraction limit. Courtesy of G.R. Musina.
-
Figure 2.
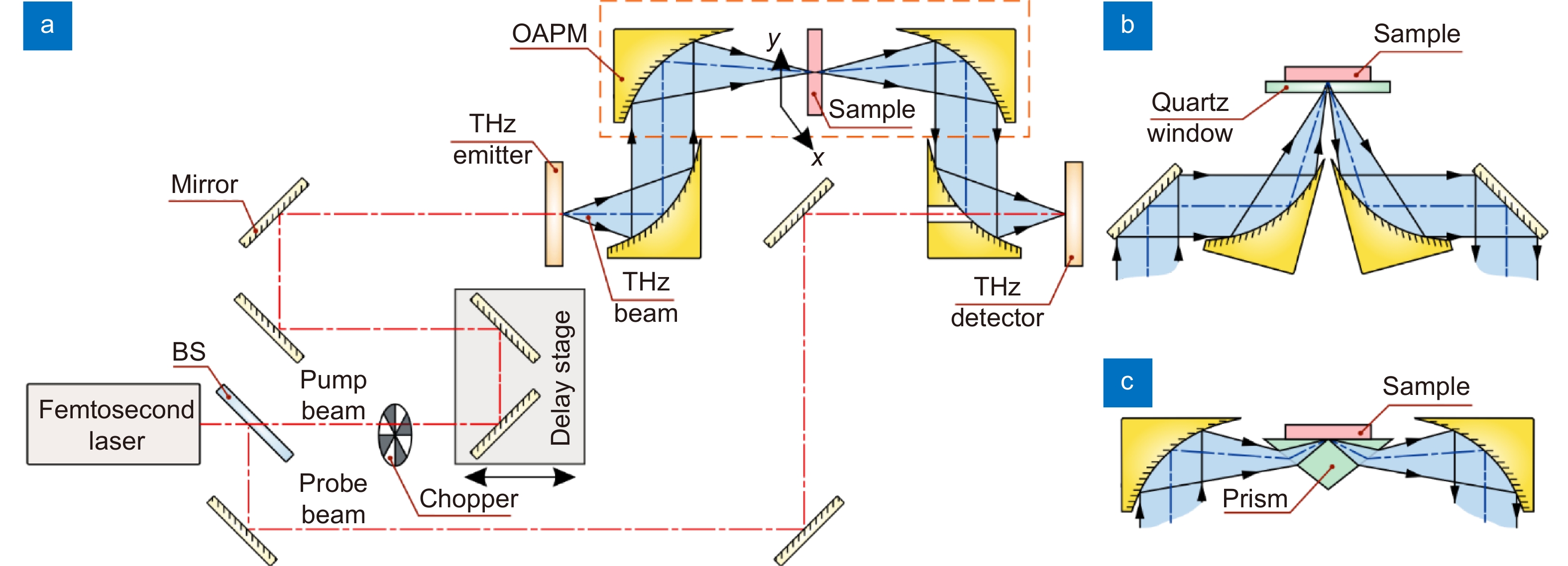
Scheme of the THz pulsed spectrometer. (a) Transmission-mode measurements. (b) Reflection-mode measurements. (c) ATR configuration. A pair of photoconductive antennas (that rely on photoconductivity/photoswitching effect), nonlinear optical crystals (that rely on optical rectification and electrooptical effects, respectively), or other principles can be used as an emitter and a detector of THz pulses. Here, BS is a beam splitter; OAPM is an off-axis parabolic mirror. Courtesy of G.R. Musina.
-
Figure 3.
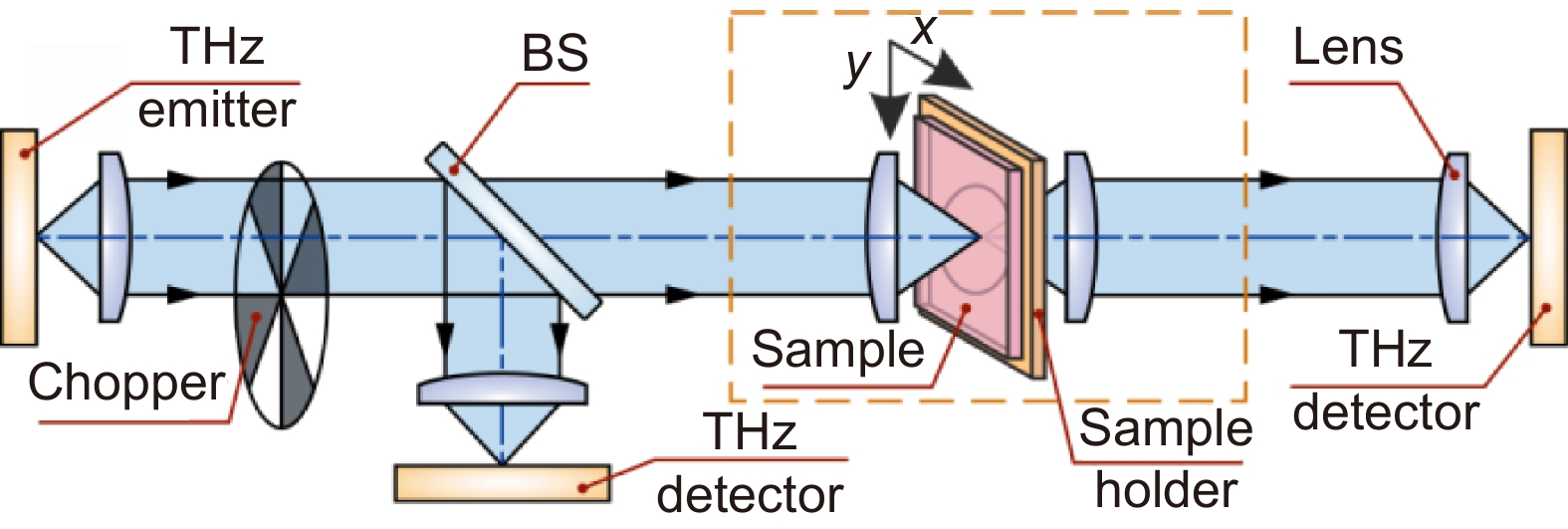
Scheme of representative THz imaging systems operating in transmission mode. Courtesy of G.R. Musina.
-
Figure 4.
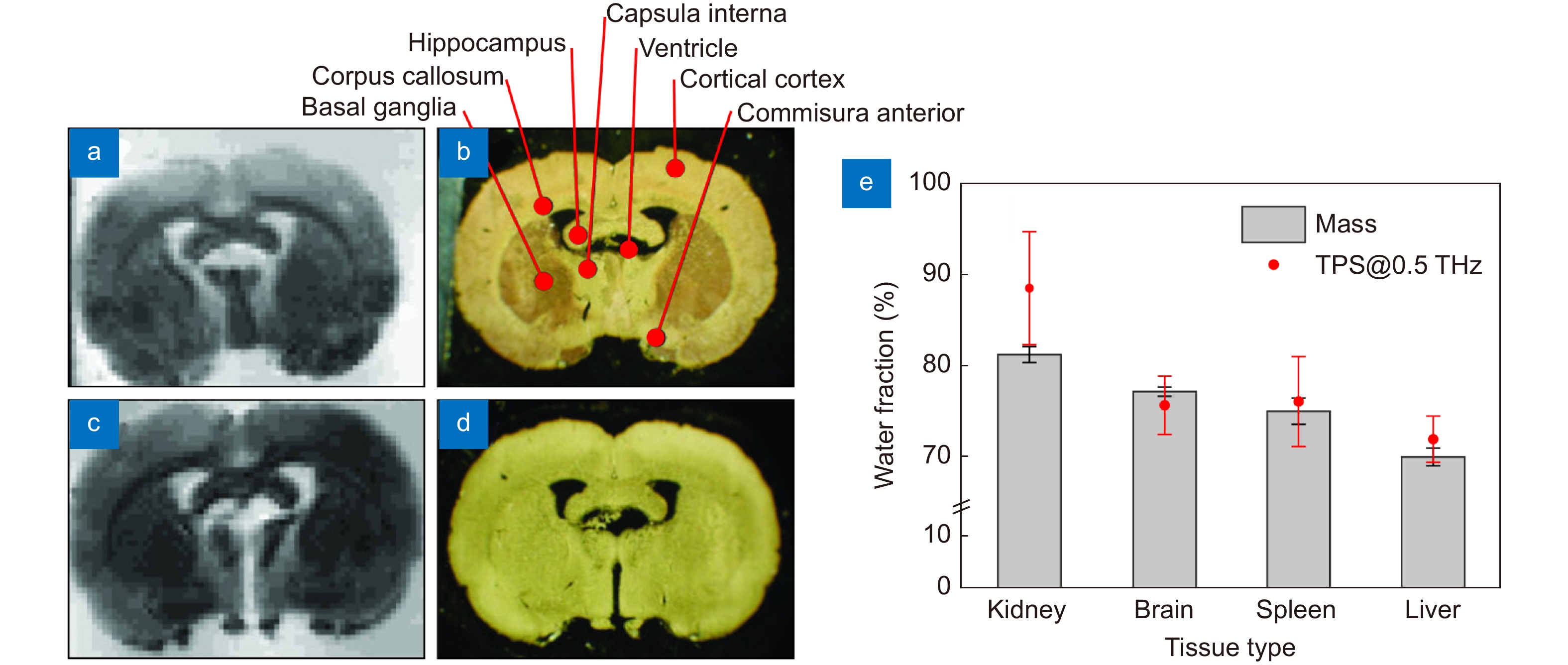
THz measurements data of intact brain ex vivo. (a–d) 3.43 THz quantum cascade laser-based images (left column) and visible morphological pictures (right column) of the frontal sections of the dehydrated rat brain ex vivo. (e) Water content in different freshy-excised (hydrated) tissues from rats ex vivo estimated based on the TPS data and tissue weighting (mass). Figure reproduced with permission from: (a) ref.81, Optical Society of America; (b) ref.58, under the OSA Open Access Publishing Agreement.
-
Figure 5.
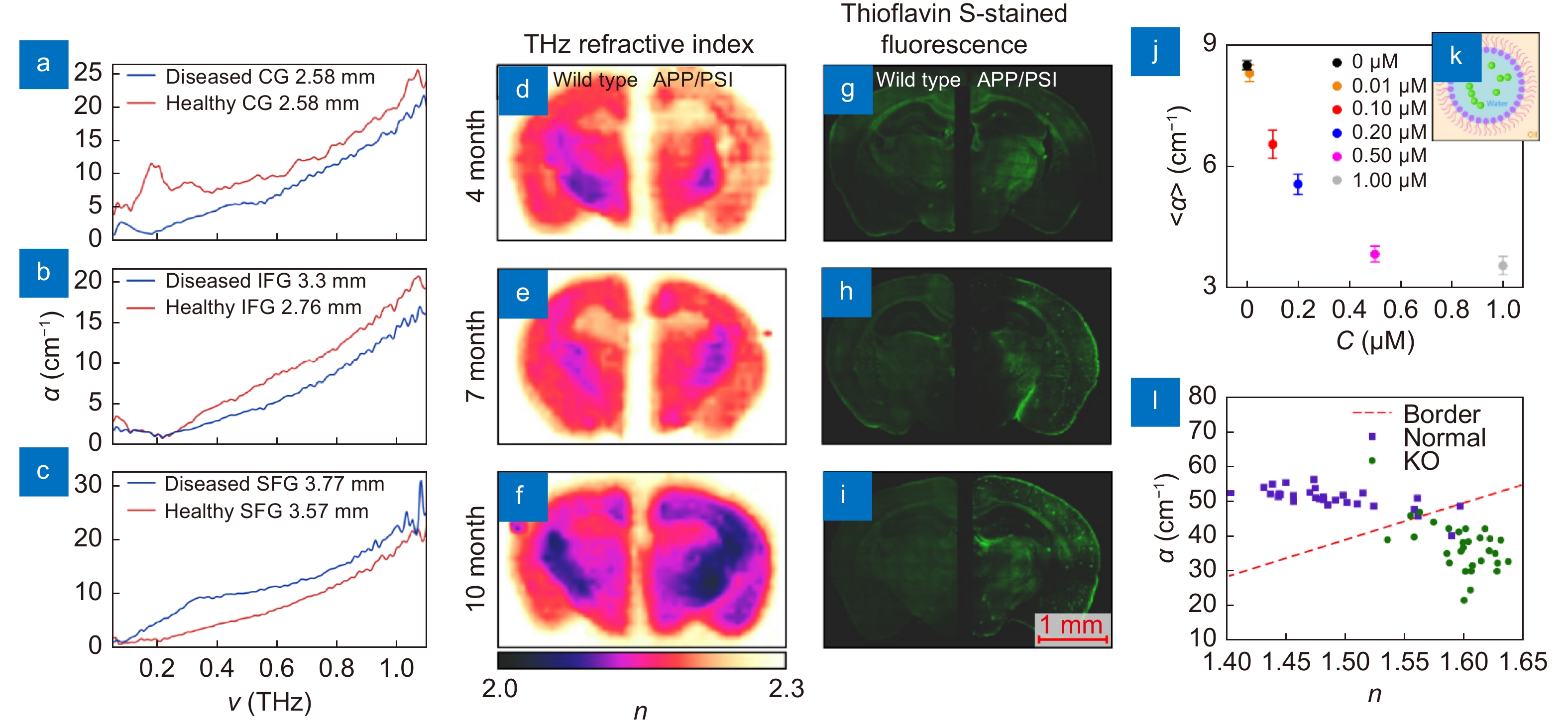
THz measurements data of the Alzheimer's and demyelinating diseases. (a–c) THz absorption coefficient α of the intact and Alzheimer's disease-altered tissues of the human brain ex vivo obtained at cryogenic temperatures using TPS, where CG, IFG, and SFG stand for the cingulate gyrus, inferior frontal gyrus, and superior frontal gyrus of the brain, respectively. (d–i) THz refractive index distributions n(r) measured by TPI (left) and Thioflavin S-stained fluorescence images (right) of the wild-type and APP/PS1 Alzheimer’s disease models depending on age. (j, k) Averaged THz absorption coefficient α of a liquid buffer with β-amyloid aggregates-containing nanodroplets, as well as the sketch of a single nanodroplet with a β-amyloid aggregate. (l) THz refractive index n and absorption coefficient α obtained from the myelin deficit (Rheb1 KO) and normal mice brains ex vivo. Figure reproduced with permission from: (a–c) ref.59, IET; (d–i) ref.140, (j, k) ref.62 Elsevier; (l) ref.68, under a Creative Commons Attribution 4.0 International License.
-
Figure 7.
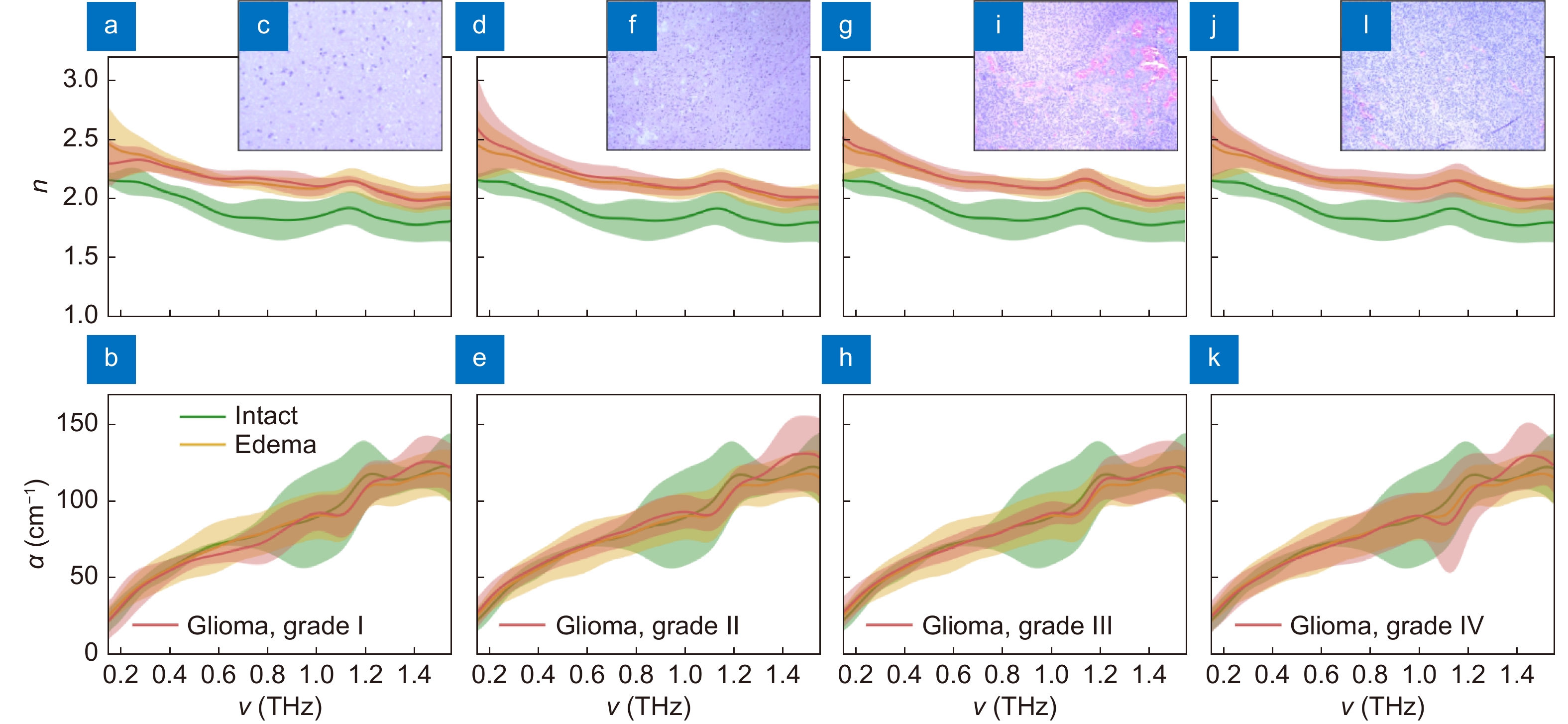
Spectra of the THz refractive index n and absorption coefficient α (by field), as well as H&E-stained histology of intact tissues, edematous tissues, and gelatin-embedded human brain gliomas ex vivo of the different WHO Grades. (a–c) Grade I. (d–f) Grade II. (g–i) Grade III. (j–l) Grade IV. THz optical properties of gliomas are compared with equal data for intact and edematous tissues, where the error bars represent a ±2.0σ confidential interval of measurements (σ is a standard deviation). Figure reproduced with permission from ref.64, under a Creative Commons Attribution 4.0 Unported License.
-
Figure 6.
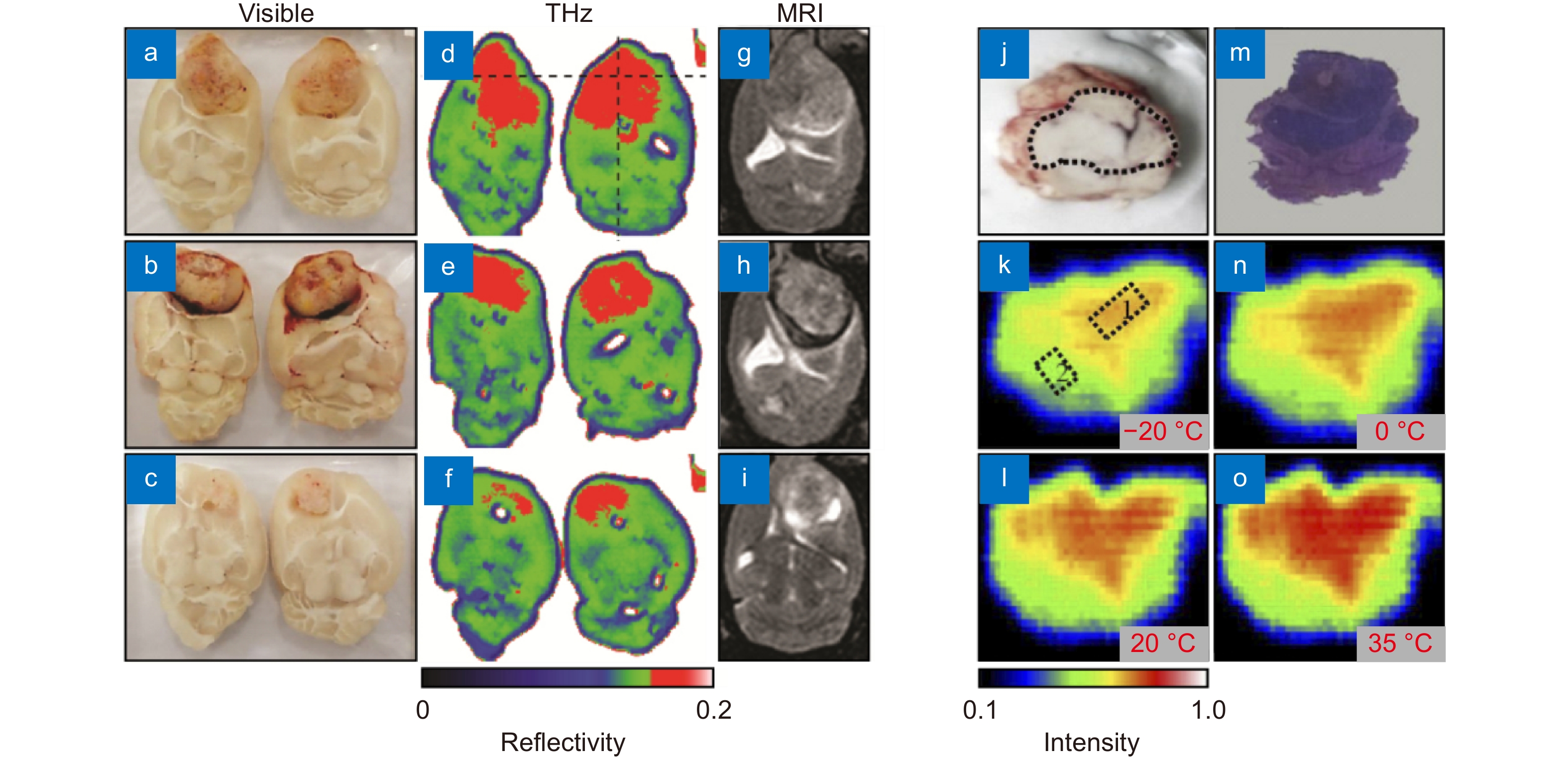
THz imaging of brain glioma models from laboratory animals ex vivo. (a–i) Visible, THz, and MRI images of orthotopic glioma model from rats, where the THz image dimensions and resolution are 4 cm×3 cm and 250 μm, respectively. (j–o) Visual image, H&E-stained histology and temperature-dependent 2.52 THz CW ATR images of orthotopic glioma model from mice ex vivo, where the sample temperature varies in the range from −20 to 35 °C. In (k–o), the blue boarder marks the boundary between background and a sample; while in (k), boxes 1 and 2 point areas of healthy and tumorous tissues. Figure reproduced with permission from: (a–i) ref.35, Optical Society of America; (j–o) ref.89, under the Optica Open Access Publishing Agreement.
-
Figure 8.
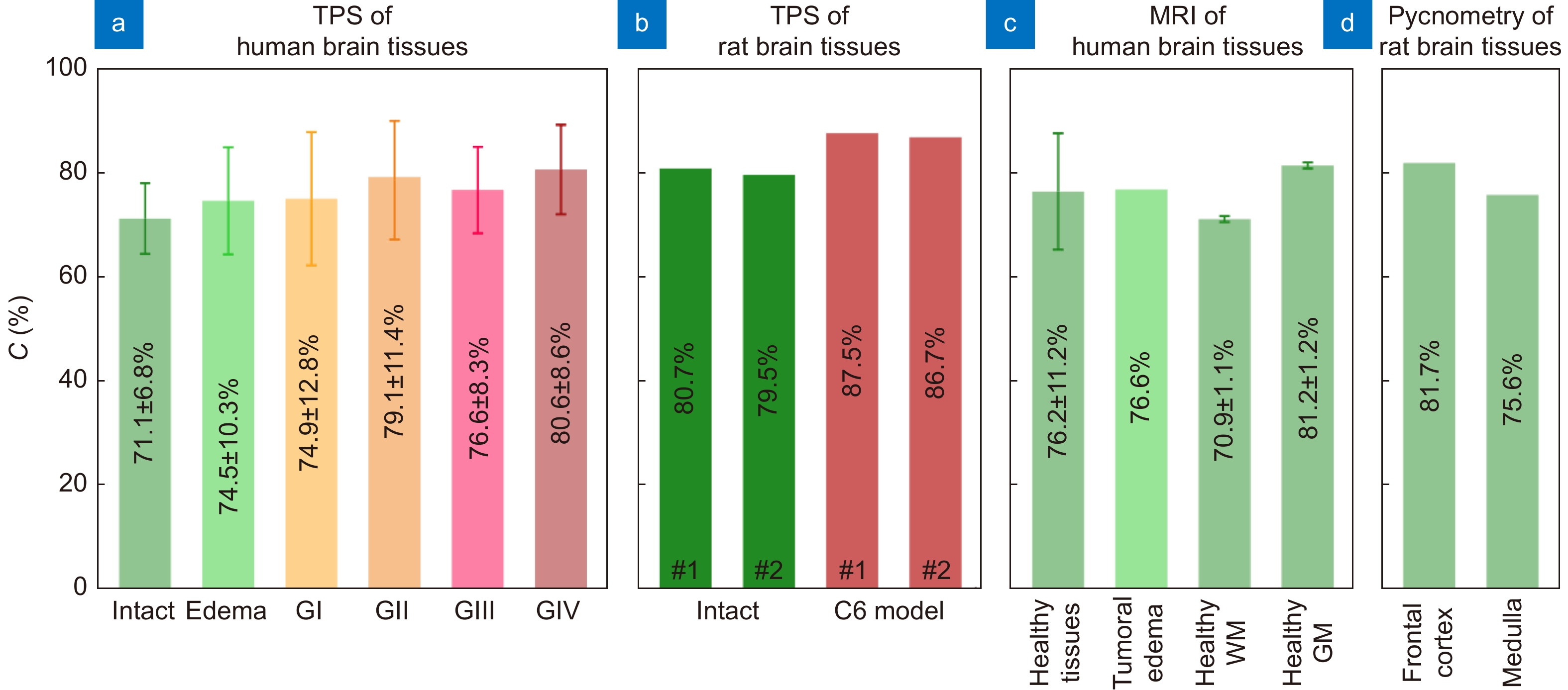
Water content in healthy and pathological tissues of the brain measured using different experimental techniques. (a) Intact tissues, edema, and WHO Grade I–IV gliomas (GI–GIV) of the human brain ex vivo measured by TPS in ref.65. (b) Intact tissues and C6 glioma model from rat brain ex vivo measured by TPS in ref.67. (c) Healthy human brain tissues and tumoral edema in vivo measured by MRI in refs.162–164, where WM and GM stand for white matter and gray matter, respectively. (d) Healthy rat brain tissues ex vivo measured by pycnometry in ref.165. Here, error bars represent fluctuations of water content within the considered set of tissue specimens. Figure reproduced with permission from ref.65, under the OSA Open Access Publishing Agreement.
-
Figure 9.
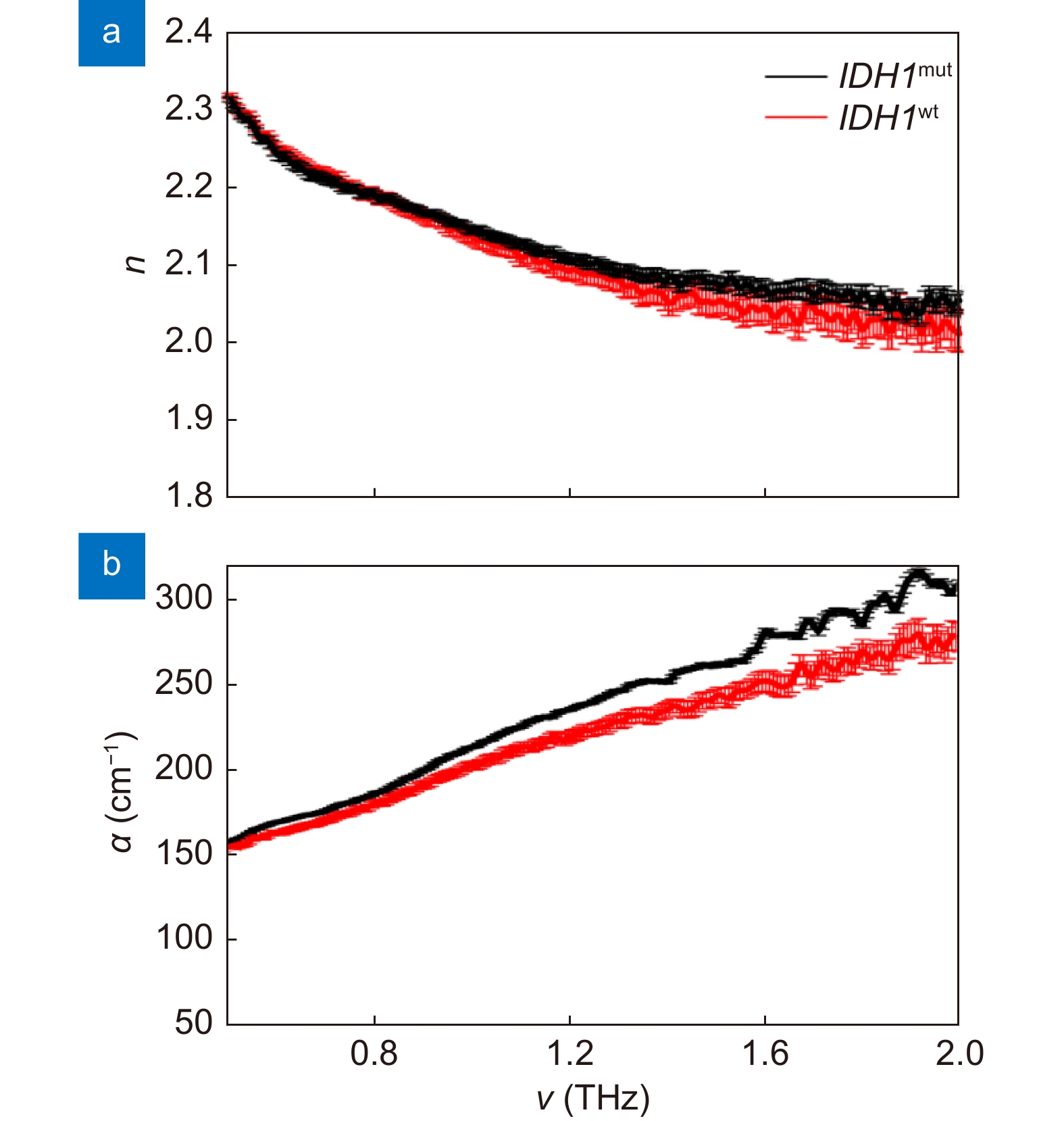
THz optical properties of IDH1 wild-type sample and IDH1 mutant positive sample. (a) Refractive index n. (b) Absorption coefficient α. Figure reproduced with permission from ref.69, under the Optica Open Access Publishing Agreement.
-
Figure 11.
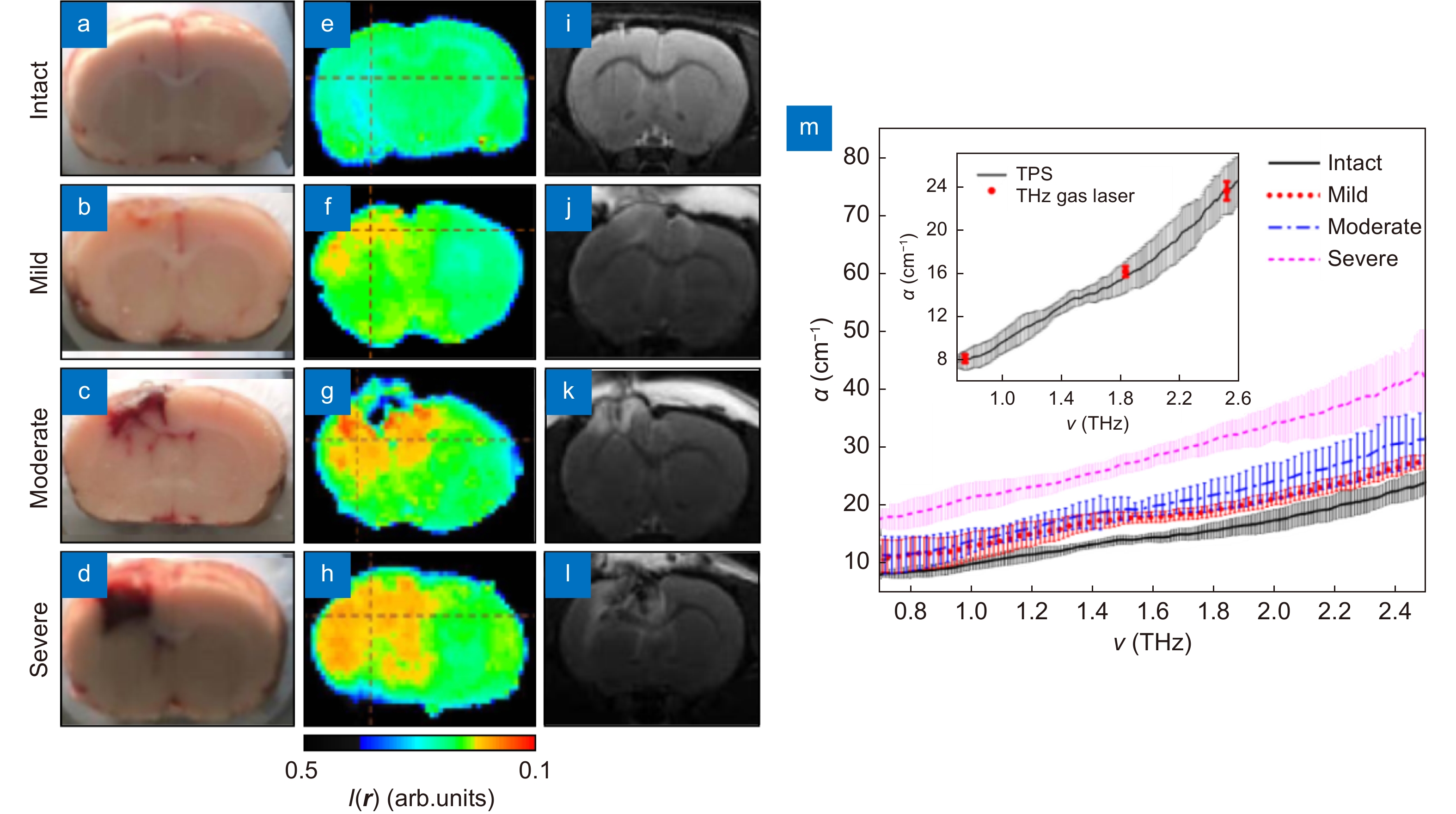
Results of the THz measurements ex vivo of the freshly-excised intact rat brain and those with TBI models of the different degrees. (a–l) Visible, THz, and MRI images of freshly-excised intact rat brain and TBI models of the mild, moderate, and severe degrees. (m) Spectra of the absorption coefficient α of the paraffin-embedded brain samples from intact rat brain and TBIs. Figure reproduced with permission from ref.83, SPIE.
-
Figure 10.
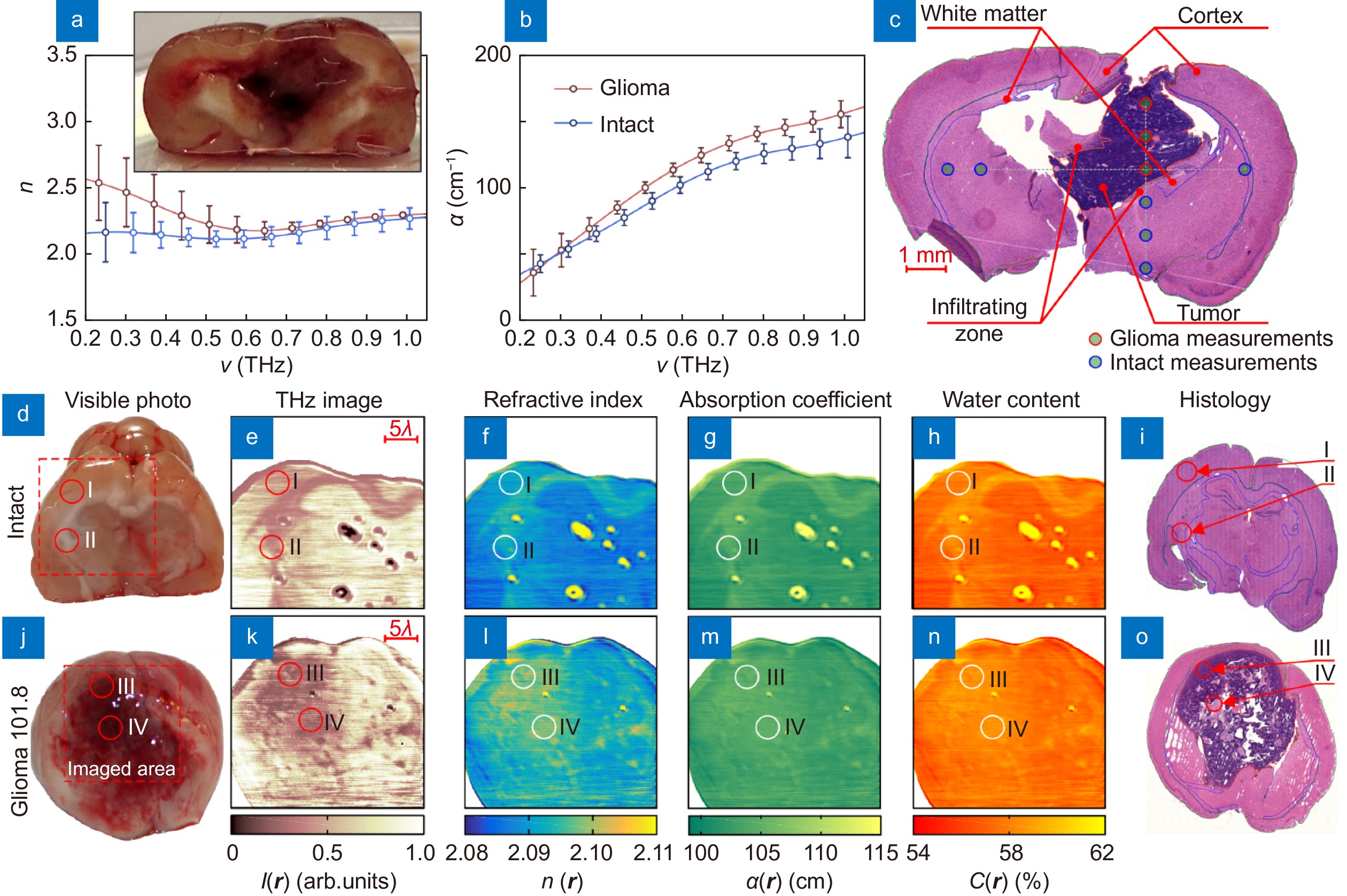
The data of TPS measurements and quantitative superresolution CW THz solid immersion microscopy (at ν = 0.6 THz or λ ≈ 500 µm) of the freshly-excised intact brain and glioma model 101.8 from rats ex vivo. (a–c) Effective THz refractive index n and absorption coefficient α (by field) of intact tissues and a tumor, measured by TPS and verified by H&E-stained histology. (d–i) Visible photo, THz image I (r), refractive index distribution n (r), absorption coefficient (by power) distribution α (r), water content distribution C (r), and H&E-stained histology, respectively, for the intact tissues. Here, r is a radius vector at the imaging plane; markers I, II point the gray matter (cortex) and white matter, respectively. (j–o) Equal data set for a tumor, where markers III, IV indicate the tumor cells accumulation and the necroses zone. Figure reproduced with permission from: (a–c) ref.116, (d–o) ref.41, under the OSA Open Access Publishing Agreement.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: