| Citation: |
|
Pencil-beam scanning catheter for intracoronary optical coherence tomography
-
Abstract
Current gradient-index (GRIN) lens based proximal-driven intracoronary optical coherence tomography (ICOCT) probes consist of a spacer and a GRIN lens with large gradient constant. This design provides great flexibility to control beam profiles, but the spacer length should be well controlled to obtain desired beam profiles and thus it sets an obstacle in mass catheter fabrication. Besides, although GRIN lens with large gradient constant can provide tight focus spot, it has short depth of focus and fast-expanded beam which leads to poor lateral resolution for deep tissue. In this paper, a type of spacer-removed probe is demonstrated with a small gradient constant GRIN lens. This design simplifies the fabrication process and is suitable for mass production. The output beam of the catheter is a narrow nearly collimated light beam, referred to as pencil beam here. The full width at half maximum beam size varies from 35.1 µm to 75.3 µm in air over 3-mm range. Probe design principles are elaborated with probe/catheter fabrication and performance test. The in vivo imaging of the catheter was verified by a clinical ICOCT system. Those results prove that this novel pencil-beam scanning catheter is potentially a good choice for ICOCT systems. -
-
References
[1] Drexler W, Fujimoto JG. Optical Coherence Tomography: Technology and Application (Springer, Germany, 2008). [2] Adler DC, Chen Y, Huber R, Schmitt J, Connolly J et al. Three-dimensional endomicroscopy using optical coherence tomography. Nat Photonics 1, 709–716 (2007). doi: 10.1038/nphoton.2007.228 [3] Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 106, 1640–1645 (2002). doi: 10.1161/01.CIR.0000029927.92825.F6 [4] Swanson EA, Fujimoto JG. The ecosystem that powered the translation of OCT from fundamental research to clinical and commercial impact [Invited]. Biomed Opt Express 8, 1638–1664 (2017). doi: 10.1364/BOE.8.001638 [5] Bouma BE, Villiger M, Otsuka K, Oh WY. Intravascular optical coherence tomography [Invited]. Biomed Opt Express 8, 2660–2686 (2017). doi: 10.1364/BOE.8.002660 [6] Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY et al. Comprehensive volumetric optical microscopy in vivo. Nat Med 12, 1429–1433 (2006). [7] Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet 390, 793–809 (2017). doi: 10.1016/S0140-6736(17)31957-8 [8] Ali ZA, Galougahi KK, Maehara A, Shlofmitz RA, Ben-Yehuda O et al. Intracoronary optical coherence tomography 2018: current status and future directions. JACC:Cardiovasc Interv 10, 2473–2487 (2017). doi: 10.1016/j.jcin.2017.09.042 [9] Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 39, 3281–3300 (2018). doi: 10.1093/eurheartj/ehy285 [10] Regar E, van Leeuwen AMGJ, Serruys PW. Optical Coherence Tomography in Cardiovascular Research (Informa Healthcare, London, 2007). [11] Schuman JS, Puliafito CA, Fujimoto JG, Duker JS. Optical Coherence Tomography of Ocular Diseases 3rd ed (Slack Incorporated, Thorofare, 2012). [12] Lu CD, Kraus MF, Potsaid B, Liu JJ, Choi W et al. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed Opt Express 5, 293–311 (2014). doi: 10.1364/BOE.5.000293 [13] Gora MJ, Suter MJ, Tearney GJ, Li XD. Endoscopic optical coherence tomography: technologies and clinical applications [Invited]. Biomed Opt Express 8, 2405–2444 (2017). doi: 10.1364/BOE.8.002405 [14] Kim TS, Park HS, Jang SJ, Song JW, Cho HS et al. Single cardiac cycle three-dimensional intracoronary optical coherence tomography. Biomed Opt Express 7, 4847–4858 (2016). doi: 10.1364/BOE.7.004847 [15] Wang TS, Pfeiffer T, Regar E, Wieser W, van Beusekom H et al. Heartbeat OCT: in vivo intravascular megahertz-optical coherence tomography. Biomed Opt Express 6, 5021–5032 (2015). doi: 10.1364/BOE.6.005021 [16] Li JN, de Groot M, Helderman F, Mo JH, Daniels JMA et al. High speed miniature motorized endoscopic probe for optical frequency domain imaging. Opt Express 20, 24132–24138 (2012). doi: 10.1364/OE.20.024132 [17] Tearney GJ, Boppart SA, Bouma BE, Brezinski ME, Weissman NJ et al. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography. Opt Lett 21, 543–545 (1996). doi: 10.1364/OL.21.000543 [18] Swanson E, Petersen CL, McNamara E, Lamport RB, Kelly DL. Ultra-small optical probes, imaging optics, and methods for using same. United States Patent: 6445939, September 3, 2002. [19] Wang W, Wang GY, Ma J, Cheng LH, Guan BO. Miniature all-fiber axicon probe with extended Bessel focus for optical coherence tomography. Opt Express 27, 358–366 (2019). doi: 10.1364/OE.27.000358 [20] Yuan W, Brown R, Mitzner W, Yarmus L, Li XD. Super-achromatic monolithic microprobe for ultrahigh-resolution endoscopic optical coherence tomography at 800 nm. Nat Commun 8, 1531 (2017). doi: 10.1038/s41467-017-01494-4 [21] Diaz-Sandoval LJ, Bouma BE, Tearney GJ, Jang IK. Optical coherence tomography as a tool for percutaneous coronary interventions. Catheter Cardio Interv 65, 492–496 (2005). doi: 10.1002/ccd.20340 [22] Qiu Y, Wang Y, Belfield KD, Liu X. Ultrathin lensed fiber-optic probe for optical coherence tomography. Biomed Opt Express 7, 2154–2162 (2016). doi: 10.1364/BOE.7.002154 [23] Moon S, Piao ZL, Kim CS, Chen ZP. Lens-free endoscopy probe for optical coherence tomography. Opt Lett 38, 2014–2016 (2013). doi: 10.1364/OL.38.002014 [24] Yin BW, Piao ZL, Nishimiya K, Hyun C, Gardecki JA et al. 3D cellular-resolution imaging in arteries using few-mode interferometry. Light:Sci Appl 8, 104 (2019). doi: 10.1038/s41377-019-0211-5 [25] Li JW, Thiele S, Quirk BC, Kirk RW, Verjans JW et al. Ultrathin monolithic 3D printed optical coherence tomography endoscopy for preclinical and clinical use. Light:Sci Appl 9, 124 (2020). doi: 10.1038/s41377-020-00365-w [26] Kim J, Xing JC, Nam HS, Song JW, Kim JW et al. Endoscopic micro-optical coherence tomography with extended depth of focus using a binary phase spatial filter. Opt Lett 42, 379–382 (2017). doi: 10.1364/OL.42.000379 [27] Durrani A, Javaid A, Lee S, Ha JY. Optical rotary junction incorporating a hollow shaft DC motor for high-speed catheter-based optical coherence tomography. Opt Lett 45, 487–490 (2020). doi: 10.1364/OL.382773 [28] Tearney GJ. Optical biopsy of in vivo tissue using optical coherence tomography (Massachusetts Institute of Technology, Cambridge, 1997). [29] Reed WA, Yan MF, Schnitzer MJ. Gradient-index fiber-optic microprobes for minimally invasive in vivo low-coherence interferometry. Opt Lett 27, 1794–1796 (2002). doi: 10.1364/OL.27.001794 [30] Mao YX, Chang SD, Sherif S, Flueraru C. Graded-index fiber lens proposed for ultrasmall probes used in biomedical imaging. Appl Opt 46, 5887–5894 (2007). doi: 10.1364/AO.46.005887 [31] Jung W, Benalcazar WA, Ahmad A, Sharma U, Tu HH et al. Numerical analysis of gradient index lens-based optical coherence tomography imaging probes. J Biomed Opt 15, 066027 (2010). doi: 10.1117/1.3523374 [32] Lorenser D, Yang X, Kirk RW, Quirk BC, McLaughlin RA et al. Ultrathin side-viewing needle probe for optical coherence tomography. Opt Lett 36, 3894–3896 (2011). doi: 10.1364/OL.36.003894 [33] Lorenser D, Yang X, Sampson DD. Accurate modeling and design of graded-index fiber probes for optical coherence tomography using the beam propagation method. IEEE Photonics J 5, 3900015 (2013). doi: 10.1109/JPHOT.2013.2250939 [34] Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science 276, 2037–2039 (1997). doi: 10.1126/science.276.5321.2037 [35] Wang LV, Wu HI. Biomedical Optics: Principles and Imaging (Wiley-Interscience, Hoboken, 2007). [36] Lee MW, Kim YH, Xing JC, Yoo HK. Astigmatism-corrected endoscopic imaging probe for optical coherence tomography using soft lithography. Opt Lett 45, 4867–4870 (2020). doi: 10.1364/OL.400383 -
Access History
Article Metrics
-
Figure 1.
Schematic diagram of the spacer-removed probe. nf, ng, nc, and nair are the refractive index of SMF core, GRIN fiber at the center, NCF, and air, respectively; lg and lc are the length of GRIN fiber and NCF; P and θ are the polishing surface and tilt angle.
-
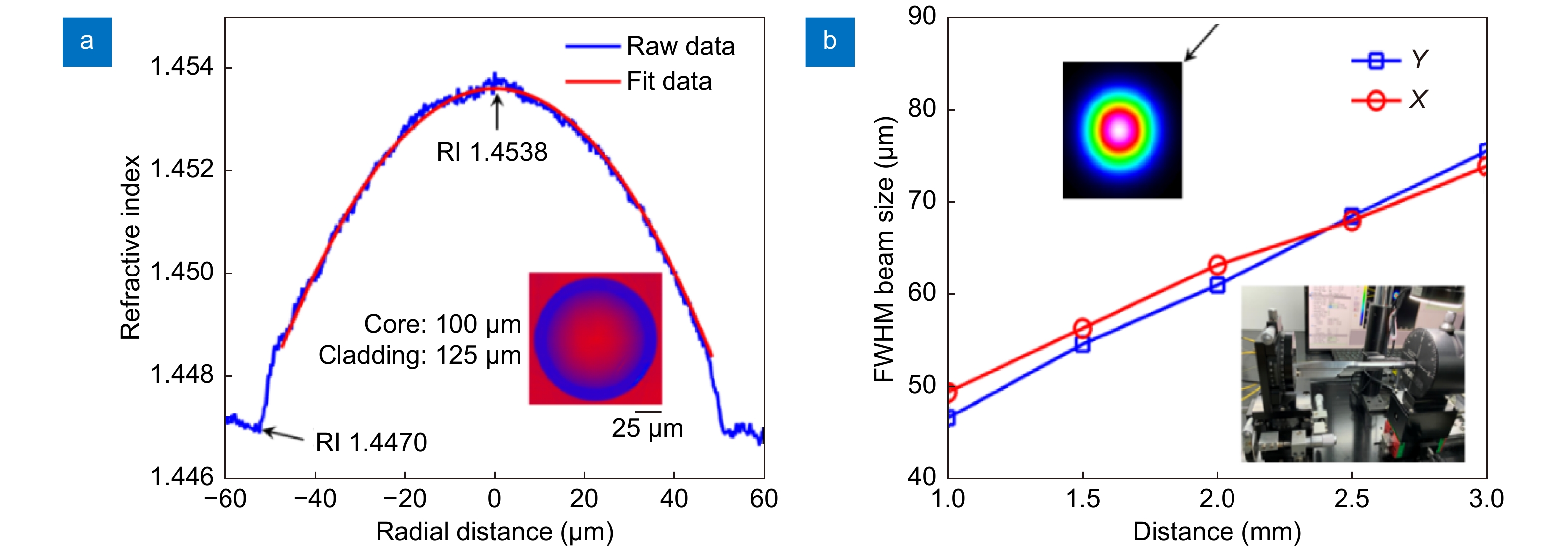
Figure 2.
(a) Refractive index of the GRIN fiber. Inset: cross section of GRIN fiber captured by the refractive index profiler. (b) FWHM beam size at different position. Inset: beam profile at 2-mm position (left top); beam measurement setup (right bottom).
-
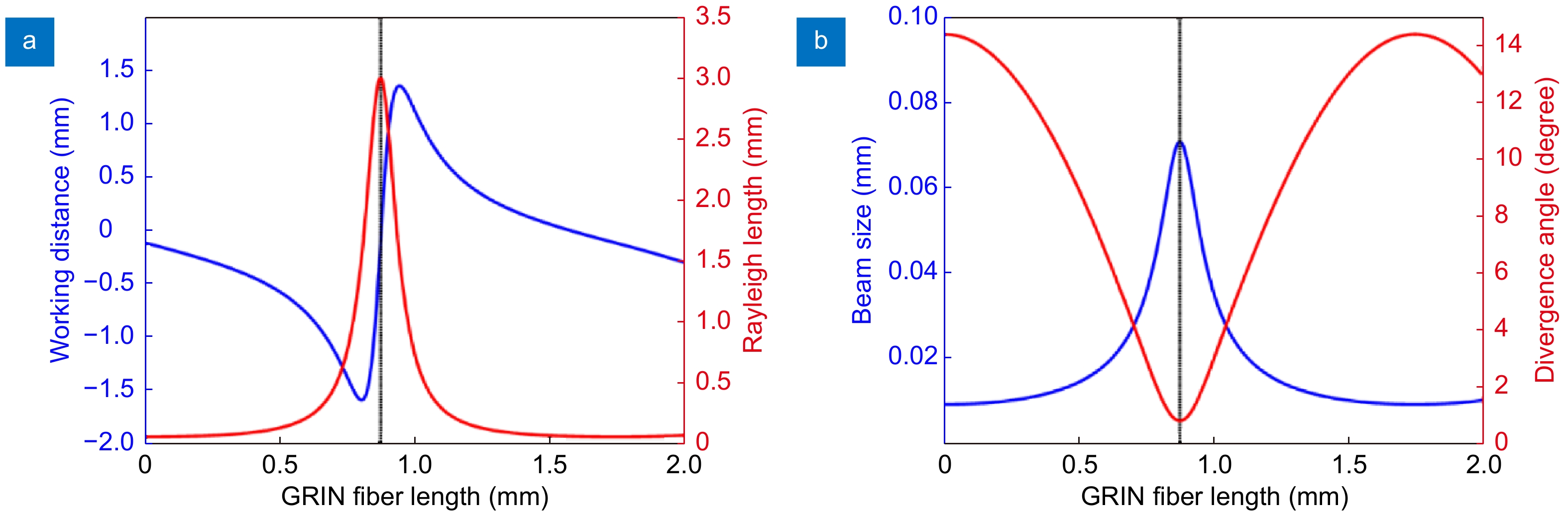
Figure 3.
(a) Working distance and Rayleigh length versus different GRIN fiber length. (b) Beam size and divergence angle versus different GRIN fiber length.
-
Figure 4.
(a) Angle-polished probe. (b) Distal optics with coil. (c) Distal image of assembled catheter with a guide wire and radiopaque marker. (d) Full view of the whole catheter. (e) Connection of the catheter and PIU. Inset: cap used to house the SC/APC connector. U: UV adhesive; C: double-wrapped torque coil; P: plastic sheath; M: radiopaque marker; G: 0.014-inch guide wire; T: catheter; L: Luer connector.
-
Figure 5.
Catheter FWHM beam size at different imaging depth. X: horizontal direction, Y: vertical direction. Inset: beam profile at 2-mm position (right bottom); beam size measurement setup (left top).
-
Figure 6.
(a) in vivo imaging arrangement. (b) X-ray image of porcine heart in this experiment. (c) Cross-section image without stent attachment. (d) Cross-section image with the stent. (e) Cutaway view of the 3D image of a segment of coronary artery with stent and guide wire. ECG: electrocardiogram; G: guidewire; H: healthy blood vessel wall; T: catheter; L: small blood vessel branch; S: stent.

 E-mail Alert
E-mail Alert RSS
RSS


 DownLoad:
DownLoad: