| Citation: | Yuan XL, Darcie T, Wei ZY, Aitchison JS. Microchip imaging cytometer: making healthcare available, accessible, and affordable. Opto-Electron Adv 5, 210130 (2022). doi: 10.29026/oea.2022.210130 |
Microchip imaging cytometer: making healthcare available, accessible, and affordable
-
Abstract
The Microchip Imaging Cytometer (MIC) is a class of integrated point-of-care detection systems based on the combination of optical microscopy and flow cytometry. MIC devices have the attributes of portability, cost-effectiveness, and adaptability while providing quantitative measurements to meet the needs of laboratory testing in a variety of healthcare settings. Based on the use of microfluidic chips, MIC requires less sample and can complete sample preparation automatically. Therefore, they can provide quantitative testing results simply using a finger prick specimen. The decreased reagent consumption and reduced form factor also help improve the accessibility and affordability of healthcare services in remote and resource-limited settings. In this article, we review recent developments of the Microchip Imaging Cytometer from the following aspects: clinical applications, microfluidic chip integration, imaging optics, and image acquisition. Following, we provide an outlook of the field and remark on promising technologies that may enable significant progress in the near future.-
Keywords:
- microchip /
- microfluidics /
- flow cytometer /
- imaging cytometer /
- biosensors /
- point-of-care testing /
- biomedical engineering
-
-
References
[1] Noor A M, Masuda T, Lei W, Horio K, Miyata Y et al. A microfluidic chip for capturing, imaging and counting CD3+ T-lymphocytes and CD19+ B-lymphocytes from whole blood. Sens Actuators B Chem 276, 107–113 (2018). doi: 10.1016/j.snb.2018.08.063 [2] Ilyas S, Sher M, Du E, Asghar W. Smartphone-based sickle cell disease detection and monitoring for point-of-care settings. Biosens Bioelectron 165, 112417 (2020). doi: 10.1016/j.bios.2020.112417 [3] Lewandowski M E. The design, fabrication, and evaluation of mobile point-of-care systems for cellular imaging in microfluidic channels. (Case Western Reserve University, Cleveland, 2018). [4] Manoto SL, Lugongolo M, Govender U, Mthunzi-Kufa P. Point of care diagnostics for HIV in resource limited settings: an overview. Medicina 54, 3 (2018). doi: 10.3390/medicina54010003 [5] Sher M, Coleman B, Caputi M, Asghar W. Development of a point-of-care assay for HIV-1 viral load using higher refractive index antibody-coated microbeads. Sensors (Basel) 21, 1819 (2021). doi: 10.3390/s21051819 [6] Qiu XB, Yang S, Wu D, Wang D, Qiao S et al. Rapid enumeration of CD4+ T lymphocytes using an integrated microfluidic system based on Chemiluminescence image detection at point-of-care testing. Biomed Microdevices 20, 1–10 (2018). doi: 10.1007/s10544-017-0241-9 [7] Min J, Chin LK, Oh J, Landeros C, Vinegoni C et al. CytoPAN—Portable cellular analyses for rapid point-of-care cancer diagnosis. Sci Transl Med 12, eaaz9746 (2020). doi: 10.1126/scitranslmed.aaz9746 [8] Nasseri B, Soleimani N, Rabiee N, Kalbasi A, Karimi M et al. Point-of-care microfluidic devices for pathogen detection. Biosens Bioelectron 117, 112–128 (2018). doi: 10.1016/j.bios.2018.05.050 [9] Zarei M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Analyt Chem 91, 26–41 (2017). doi: 10.1016/j.trac.2017.04.001 [10] Ymeti A, Li X, Lunter B, Breukers C, Tibbe AGJ et al. A single platform image cytometer for resource‐poor settings to monitor disease progression in HIV infection. Cytometry Part A 71, 132–142 (2007). [11] Spijkerman R, Hesselink L, Hellebrekers P, Vrisekoop N, Hietbrink F et al. Automated flow cytometry enables high performance point-of-care analysis of leukocyte phenotypes. J Immunol Methods 474, 112646 (2019). doi: 10.1016/j.jim.2019.112646 [12] Kestens L, Mandy F. Thirty‐five years of CD4 T‐cell counting in HIV infection: From flow cytometry in the lab to point-of-care testing in the field. Cytometry Part B Clin Cytom 92, 437–444 (2017). doi: 10.1002/cyto.b.21400 [13] Knowlton S, Joshi A, Syrrist P, Coskun A F, Tasoglu S. 3D-printed smartphone-based point of care tool for fluorescence-and magnetophoresis-based cytometry. Lab Chip 17, 2839–2851 (2017). doi: 10.1039/C7LC00706J [14] Hassan U, Ghonge T, Reddy B Jr, Patel M, Rappleye M et al. A point-of-care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat Commun 8, 15949 (2017). doi: 10.1038/ncomms15949 [15] Shapiro HM, Perlmutter NG. Personal cytometers: slow flow or no flow. Cytometry Part A 69, 620–630 (2006). [16] Zhang S, Liu YY, Wang XF, Yang L, Li HS et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol 13, 120 (2020). doi: 10.1186/s13045-020-00954-7 [17] Cossarizza A, De Biasi S, Guaraldi G, Girardis M, Mussini C et al. SARS-CoV-2, the virus that causes COVID-19: Cytometry and the new challenge for global health. Cytometry 97, 340–343 (2020). doi: 10.1002/cyto.a.24002 [18] Bransky A, Larsson A, Aardal E, Ben-Yosef Y, Christenson RH. A novel approach to hematology testing at the point of care. J Appl Lab Med 6, 532–542 (2021). doi: 10.1093/jalm/jfaa186 [19] Spijkerman R, Bongers SH, Bindels BJ, Tinnevelt GH, Giustarini G et al. Flow cytometric evaluation of the neutrophil compartment in COVID‐19 at hospital presentation: A normal response to an abnormal situation. J Leukoc Biol 109, 99–114 (2021). doi: 10.1002/JLB.5COVA0820-520RRR [20] Larsen CH. The fragile environments of inexpensive CD4+ T‐cell enumeration in the least developed countries: Strategies for accessible support. Cytometry Part B Clin Cytom 74, S107–S116 (2008). [21] Janossy G, Shapiro H. Simplified cytometry for routine monitoring of infectious diseases. Cytometry Part B Clin Cytom 74, S6–S10 (2008). [22] Shapiro HM, Perlmutter NG. Killer applications: Toward affordable rapid cell‐based diagnostics for malaria and tuberculosis. Cytometry Part B Clin Cytom 74, S152–S164 (2008). [23] Marinucci F, Medina‐Moreno S, Paterniti AD, Wattleworth M, Redfield RR. Decentralization of CD4 testing in resource‐limited settings: 7 years of experience in six African countries. Cytometry Part A 79, 368–374 (2011). [24] Logan C, Givens M, Ives JT, Delaney M, Lochhead MJ et al. Performance evaluation of the MBio Diagnostics point-of-care CD4 counter. J Immunol Methods 387, 107–113 (2013). doi: 10.1016/j.jim.2012.10.002 [25] Mbopi-Kéou FX, Sagnia B, Ngogang J, Angwafo III FF, Colizzi V et al. Validation of a single-platform, volumetric, flow cytometry for CD4 T cell count monitoring in therapeutic mobile unit. J Transl Med 10, 22 (2012). doi: 10.1186/1479-5876-10-22 [26] Piyasena ME, Graves SW. The intersection of flow cytometry with microfluidics and microfabrication. Lab Chip 14, 1044–1059 (2014). doi: 10.1039/C3LC51152A [27] Spencer D, Caselli F, Bisegna P, Morgan H. High accuracy particle analysis using sheathless microfluidic impedance cytometry. Lab Chip 16, 2467–2473 (2016). doi: 10.1039/C6LC00339G [28] Leary JF. Design of portable microfluidic cytometry devices for rapid medical diagnostics in the field. Proc SPIE 10881, 108810B (2019). [29] Vázquez RM, Trotta G, Paturzo M, Volpe A, Bernava G et al. Imaging cytometry in a plastic ultra-mobile system. Proc SPIE 10055, 100550H (2017). [30] Guo C, Liu XM, Kan XC, Zhang FL, Tan JB et al. Lensfree on-chip microscopy based on dual-plane phase retrieval. Opt Express 27, 35216–35229 (2019). doi: 10.1364/OE.27.035216 [31] Gong YL, Fan N, Yang X, Peng B, Jiang H. New advances in microfluidic flow cytometry. Electrophoresis 40, 1212–1229 (2019). doi: 10.1002/elps.201800298 [32] Lei C, Kobayashi H, Wu Y, Li M, Isozaki A et al. High-throughput imaging flow cytometry by optofluidic time-stretch microscopy. Nat Protoc 13, 1603–1631 (2018). doi: 10.1038/s41596-018-0008-7 [33] Rane AS, Rutkauskaite J, deMello A, Stavrakis S. High-throughput multi-parametric imaging flow cytometry. Chem 3, 588–602 (2017). doi: 10.1016/j.chempr.2017.08.005 [34] Kamentsky LA. Future directions for flow cytometry. J Histochem Cytochem 27, 1649–1654 (1979). doi: 10.1177/27.12.391998 [35] Cambier JL, Kay DB, Wheeless LL Jr. A multidimensional slit-scan flow system. J Histochem Cytochem 27, 321–324 (1979). doi: 10.1177/27.1.374595 [36] Kachel V, Benker G, Lichtnau K, Valet G, Glossner E. Fast imaging in flow: a means of combining flow-cytometry and image analysis. J Histochem Cytochem 27, 335–341 (1979). doi: 10.1177/27.1.374598 [37] Barteneva NS, Fasler-Kan E, Vorobjev IA. Imaging flow cytometry: coping with heterogeneity in biological systems. J Histochem Cytochem 60, 723–733 (2012). doi: 10.1369/0022155412453052 [38] Doan M, Vorobjev I, Rees P, Filby A, Wolkenhauer O et al. Diagnostic potential of imaging flow cytometry. Trends Biotechnol 36, 649–652 (2018). doi: 10.1016/j.tibtech.2017.12.008 [39] Stavrakis S, Holzner G, Choo J, DeMello A. High-throughput microfluidic imaging flow cytometry. Curr Opin Biotechnol 55, 36–43 (2019). doi: 10.1016/j.copbio.2018.08.002 [40] Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol 33, 692–705 (2015). doi: 10.1016/j.tibtech.2015.09.001 [41] Dincer C, Bruch R, Costa‐Rama E, Fernández‐Abedul MT, Merkoçi A et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv Mater 31, 1806739 (2019). [42] Dincer C, Bruch R, Kling A, Dittrich PS, Urban GA. Multiplexed point-of-care testing–xPOCT. Trends Biotechnol 35, 728–742 (2017). doi: 10.1016/j.tibtech.2017.03.013 [43] Rong Z, Wang Q, Sun NX, Jia XF, Wang KL et al. Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal Chim Acta 1055, 140–147 (2019). doi: 10.1016/j.aca.2018.12.043 [44] Liu JJ, Geng ZX, Fan ZY, Liu J, Chen HD. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens Bioelectron 132, 17–37 (2019). doi: 10.1016/j.bios.2019.01.068 [45] Xu DD, Huang XW, Guo JH, Ma X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens Bioelectron 110, 78–88 (2018). doi: 10.1016/j.bios.2018.03.018 [46] Lin BX, Yu Y, Cao YJ, Guo ML, Zhu DB et al. Point-of-care testing for streptomycin based on aptamer recognizing and digital image colorimetry by smartphone. Biosens Bioelectron 100, 482–489 (2018). doi: 10.1016/j.bios.2017.09.028 [47] Zhang JJ, Shen Z, Xiang Y, Lu Y. Integration of solution-based assays onto lateral flow device for one-step quantitative point-of-care diagnostics using personal glucose meter. ACS Sens 1, 1091–1096 (2016). doi: 10.1021/acssensors.6b00270 [48] Hu J, Cui XY, Gong Y, Xu XY, Gao B et al. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol Adv 34, 305–320 (2016). doi: 10.1016/j.biotechadv.2016.02.008 [49] Chen YT, Cheng N, Xu YC, Huang KL, Luo YB et al. Point-of-care and visual detection of P. aeruginosa and its toxin genes by multiple LAMP and lateral flow nucleic acid biosensor. Biosens Bioelectron 81, 317–323 (2016). doi: 10.1016/j.bios.2016.03.006 [50] Gong Y, Zheng YM, Jin BR, You ML, Wang JY et al. A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 201, 126–133 (2019). doi: 10.1016/j.talanta.2019.03.105 [51] Kim H, Chung DR, Kang M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst 144, 2460–2466 (2019). doi: 10.1039/C8AN02295J [52] Choi JR, Hu J, Gong Y, Feng SS, Abas WABW et al. An integrated lateral flow assay for effective DNA amplification and detection at the point of care. Analyst 141, 2930–2939 (2016). doi: 10.1039/C5AN02532J [53] Li ZD, Bai YM, You ML, Hu J, Yao CY et al. Fully integrated microfluidic devices for qualitative, quantitative and digital nucleic acids testing at point of care. Biosens Bioelectron 177, 112952 (2021). doi: 10.1016/j.bios.2020.112952 [54] Gou T, Hu JM, Wu WS, Ding X, Zhou SF et al. Smartphone-based mobile digital PCR device for DNA quantitative analysis with high accuracy. Biosens Bioelectron 120, 144–152 (2018). doi: 10.1016/j.bios.2018.08.030 [55] Cao L, Guo XJ, Mao P, Ren YL, Li ZD et al. A Portable Digital Loop-Mediated Isothermal Amplification Platform Based on Microgel Array and Hand-Held Reader. ACS Sens 6, 3564–3574 (2021). doi: 10.1021/acssensors.1c00603 [56] Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low-and middle-income countries. PLoS Med 9, e1001306 (2012). doi: 10.1371/journal.pmed.1001306 [57] Mahoney E, Kun J, Smieja M, Fang QY. Review-point-of-care urinalysis with emerging sensing and imaging technologies. J Electrochem Soc 167, 037518 (2019). [58] Olanrewaju AO, Ng A, DeCorwin-Martin P, Robillard A, Juncker D. Microfluidic capillaric circuit for rapid and facile bacteria detection. Anal Chem 89, 6846–6853 (2017). doi: 10.1021/acs.analchem.7b01315 [59] Zhang YS, Watts BR, Guo TY, Zhang ZY, Xu CQ et al. Optofluidic device based microflow cytometers for particle/cell detection: a review. Micromachines (Basel) 7, 70 (2016). doi: 10.3390/mi7040070 [60] Dou JJ, Aitchison JS, Chen L, Nayyar R. Portable point-of-care blood analysis system for global health (Conference Presentation). Proc SPIE 9699, 96990M (2016). [61] Dou JJ. A miniaturized microfluidic cytometer platform for point-of-care blood testing applications. (University of Toronto, Toronto, 2017). [62] Dou J, Chen L, Nayyar R, Aitchison JS. A miniaturized particle detection system for HIV monitoring. In 2013 IEEE Photonics Conference 5–7 (IEEE, 2013);http://doi.org/10.1109/IPCon.2013.6656338. [63] Dou J, Chen L, Nayyar R, Aitchison S. A microfluidic based optical particle detection method. Proc SPIE 8229, 82290B (SPIE, 2012). [64] Yuan XL, Darcie T, McKendry JJD, Dawson MD, Strain MJ et al. LED Excitation of an Imaging Cytometer for Bead-based Immunoassay. IEEE Photon Technol Lett 33, 892–895 (2021). doi: 10.1109/LPT.2021.3070079 [65] Yuan XL, Garg S, De Haan K, Fellouse FA, Gopalsamy A et al. Bead-based multiplex detection of dengue biomarkers in a portable imaging device. Biomed Opt Express 11, 6154–6167 (2020). doi: 10.1364/BOE.403803 [66] Garg S, Yuan RX, Gopalsamy A, Fellouse FA, Sidhu SS et al. A multiplexed, point-of-care sensing for dengue In 2019 IEEE SENSORS 1–4 (IEEE, 2019);http://doi.org/10.1109/SENSORS43011.2019.8956616. [67] Kanakasabapathy MK, Sadasivam M, Singh A, Preston C, Thirumalaraju P et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis. Sci Transl Med 9, eaai7863 (2017). doi: 10.1126/scitranslmed.aai7863 [68] Gordon P, Venancio VP, Mertens-Talcott SU, Coté GL. Portable bright-field, fluorescence, and cross-polarized microscope toward point-of-care imaging diagnostics. J Biomed Opt 24, 096502 (2019). doi: 10.1117/1.JBO.24.9.096502 [69] Zhu HY, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal. Chem 83, 6641–6647 (2011). doi: 10.1021/ac201587a [70] Wei QS, McLeod E, Qi HF, Wan Z, Sun R et al. On-chip cytometry using plasmonic nanoparticle enhanced lensfree holography. Sci Rep 3, 1699 (2013). doi: 10.1038/srep01699 [71] De Haan K, Koydemir HC, Rivenson Y, Tseng D, Van Dyne E et al. Automated screening of sickle cells using a smartphone-based microscope and deep learning. NPJ Digit Med 3, 1–9 (2020). doi: 10.1038/s41746-019-0211-0 [72] Dekker S, Buesink W, Blom M, Alessio M, Verplanck N et al. Standardized and modular microfluidic platform for fast Lab on Chip system development. Sens Actuators B Chem 272, 468–478 (2018). doi: 10.1016/j.snb.2018.04.005 [73] Fan YQ, Wang HL, Gao KX, Liu JJ, Chai DP et al. Applications of modular microfluidics technology. Chin J Anal Chem 46, 1863–1871 (2018). doi: 10.1016/S1872-2040(18)61126-0 [74] Lee TY, Han K, Barrett DO, Park S, Soper SA et al. Accurate, predictable, repeatable micro-assembly technology for polymer, microfluidic modules. Sens Actuators B Chem 254, 1249–1258 (2018). doi: 10.1016/j.snb.2017.07.189 [75] Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med 7, 273re1 (2015). doi: 10.1126/scitranslmed.aaa0056 [76] Ghonge T, Koydemir HC, Valera E, Berger J, Garcia C et al. Smartphone-imaged microfluidic biochip for measuring CD64 expression from whole blood. Analyst 144, 3925–3935 (2019). doi: 10.1039/C9AN00532C [77] Poudineh M, Maikawa CL, Ma EY, Pan J, Mamerow D et al. A fluorescence sandwich immunoassay for the real-time continuous detection of glucose and insulin in live animals. Nat Biomed Eng 5, 53–63 (2021). doi: 10.1038/s41551-020-00661-1 [78] Calero V, Garcia-Sanchez P, Honrado C, Ramos A, Morgan H. AC electrokinetic biased deterministic lateral displacement for tunable particle separation. Lab Chip 19, 1386–1396 (2019). doi: 10.1039/C8LC01416G [79] Richards JR, Gaylor KA, Pilgrim AJ. Comparison of traditional otoscope to iPhone otoscope in the pediatric ED. Am J Emerg Med 33, 1089–1092 (2015). doi: 10.1016/j.ajem.2015.04.063 [80] Dunning K, Stothard JR. From the McArthur to the millennium health microscope (MHM): future developments in microscope miniaturization for international health. Micros Today 15, 18–21 (2007). [81] Jones D, Nyalwidhe J, Tetley L, Barrett MP. McArthur revisited: fluorescence microscopes for field diagnostics. Trends Parasitol 23, 468–469 (2007). doi: 10.1016/j.pt.2007.08.003 [82] Chen H, Li Z, Zhang LZ, Sawaya P, Shi JB et al. Quantitation of femtomolar‐level protein biomarkers using a simple microbubbling digital assay and bright‐field smartphone imaging. Angew Chem 131, 14060–14066 (2019). doi: 10.1002/ange.201906856 [83] Jagannadh VK, Adhikari JV, Gorthi SS. Automated cell viability assessment using a microfluidics based portable imaging flow analyzer. Biomicrofluidics 9, 024123 (2015). doi: 10.1063/1.4919402 [84] Shcheslavskiy VI, Neubauer A, Bukowiecki R, Dinter F, Becker W. Combined fluorescence and phosphorescence lifetime imaging. Applied Physics Letters 108, 091111 (2016). [85] Huang L, Tian SL, Zhao WH, Liu K, Ma X et al. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst 145, 2828–2840 (2020). doi: 10.1039/C9AN02485A [86] Graham H, Chandler DJ, Dunbar SA. The genesis and evolution of bead-based multiplexing. Methods 158, 2–11 (2019). doi: 10.1016/j.ymeth.2019.01.007 [87] Miyazaki CM, Kinahan DJ, Mishra R, Mangwanya F, Kilcawley N et al. Label-free, spatially multiplexed SPR detection of immunoassays on a highly integrated centrifugal Lab-on-a-Disc platform. Biosens Bioelectron 119, 86–93 (2018). doi: 10.1016/j.bios.2018.07.056 [88] Ming K, Kim J, Biondi MJ, Syed A, Chen K et al. Integrated quantum dot barcode smartphone optical device for wireless multiplexed diagnosis of infected patients. ACS Nano 9, 3060–74 (2015). doi: 10.1021/nn5072792 [89] Chia YH, Yeh JA, Huang YY, Luo Y. Simultaneous multi-color optical sectioning fluorescence microscopy with wavelength-coded volume holographic gratings. Opt Express 28, 37177–37187 (2020). doi: 10.1364/OE.409179 [90] Kage D, Hoffmann K, Borcherding H, Schedler U, Resch-Genger U. Lifetime encoding in flow cytometry for bead-based sensing of biomolecular interaction. Sci Rep 10, 19477 (2020). doi: 10.1038/s41598-020-76150-x [91] Datta R, Heaster TM, Sharick JT, Gillette AA, Skala MC. Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. J Biomed Opt 25, 071203 (2020). [92] Cordina NM, Sayyadi N, Parker LM, Everest-Dass A, Brown LJ et al. Reduced background autofluorescence for cell imaging using nanodiamonds and lanthanide chelates. Sci Rep 8, 4521 (2018). doi: 10.1038/s41598-018-22702-1 [93] Zhang J, Shikha S, Mei QS, Liu JL, Zhang Y. Fluorescent microbeads for point-of-care testing: a review. Microchim Acta 186, 361 (2019). doi: 10.1007/s00604-019-3449-y [94] Becker W. Advanced Time-Correlated Single Photon Counting Techniques (Springer, Berlin Heidelberg, 2005). [95] Wei LP, Yan WR, Ho D. Recent advances in fluorescence lifetime analytical microsystems: Contact optics and CMOS time-resolved electronics. Sensors (Basel) 17, 2800 (2017). doi: 10.3390/s17122800 [96] Han YY, Gu Y, Zhang AC, Lo YH. Imaging technologies for flow cytometry. Lab Chip 16, 4639–4647 (2016). doi: 10.1039/C6LC01063F [97] Lu GL, Fei BW. Medical hyperspectral imaging: a review. J Biomed Opt 19, 010901 (2014). doi: 10.1117/1.JBO.19.1.010901 [98] Signoroni A, Savardi M, Baronio A, Benini S. Deep learning meets hyperspectral image analysis: A multidisciplinary review. J Imaging 5, 52 (2019). doi: 10.3390/jimaging5050052 [99] Seo S, Su TW, Tseng DK, Erlinger A, Ozcan A. Lensfree holographic imaging for on-chip cytometry and diagnostics. Lab Chip 9, 777–787 (2009). doi: 10.1039/B813943A [100] Su TW, Seo S, Erlinger A, Ozcan A. High‐throughput lensfree imaging and characterization of a heterogeneous cell solution on a chip. Biotechnol Bioeng 102, 856–868 (2009). doi: 10.1002/bit.22116 [101] Mudanyali O, Tseng D, Oh C, Isikman SO, Sencan I et al. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip 10, 1417–1428 (2010). doi: 10.1039/c000453g [102] Kim SB, Bae H, Koo KI, Dokmeci MR, Ozcan A et al. Lens-free imaging for biological applications. J Lab Autom 17, 43–49 (2012). doi: 10.1177/2211068211426695 [103] Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip 8, 98–106 (2008). doi: 10.1039/B713695A [104] Ah Lee S, Leitao R, Zheng GA, Yang S, Rodriguez A et al. Color capable sub-pixel resolving optofluidic microscope and its application to blood cell imaging for malaria diagnosis. PLoS One 6, e26127 (2011). doi: 10.1371/journal.pone.0026127 [105] Ah Lee S, Yang C. A smartphone-based chip-scale microscope using ambient illumination. Lab Chip 14, 3056–3063 (2014). doi: 10.1039/C4LC00523F [106] Tseng D, Mudanyali O, Oztoprak C, Isikman SO, Sencan I et al. Lensfree microscopy on a cellphone. Lab Chip 10, 1787–1792 (2010). doi: 10.1039/c003477k [107] Im H, Castro CM, Shao HL, Liong M, Song J et al. Digital diffraction analysis enables low-cost molecular diagnostics on a smartphone. Proc Natl Acad Sci USA 112, 5613–5618 (2015). doi: 10.1073/pnas.1501815112 [108] Mariën J, Stahl R, Lambrechts A, Van Hoof C, Yurt A. Color lens-free imaging using multi-wavelength illumination based phase retrieval. Opt Express 28, 33002–33018 (2020). doi: 10.1364/OE.402293 [109] Goda K, Tsia KK, Jalali B. Serial time-encoded amplified imaging for real-time observation of fast dynamic phenomena. Nature 458, 1145–1149 (2009). doi: 10.1038/nature07980 [110] Basiji DA, Ortyn WE, Liang LC, Venkatachalam V, Morrissey P. Cellular image analysis and imaging by flow cytometry. Clin Lab Med 27, 653–670 (2007). doi: 10.1016/j.cll.2007.05.008 [111] Hu WT, Soper SA, Jackson JM. Time-delayed integration–spectral flow cytometer (TDI-SFC) for low-abundance-cell immunophenotyping. Anal Chem 91, 4656–4664 (2019). doi: 10.1021/acs.analchem.9b00021 [112] Schonbrun E, Gorthi SS, Schaak D. Microfabricated multiple field of view imaging flow cytometry. Lab Chip 12, 268–273 (2012). doi: 10.1039/C1LC20843H [113] Gorthi SS, Schaak D, Schonbrun E. Fluorescence imaging of flowing cells using a temporally coded excitation. Opt Express 21, 5164–5170 (2013). doi: 10.1364/OE.21.005164 [114] Ho D, Noor MO, Krull UJ, Gulak G, Genov R. CMOS tunable-color image sensor with dual-ADC shot-noise-aware dynamic range extension. IEEE Trans Circ Syst I Regular Papers 60, 2116–2129 (2013). doi: 10.1109/TCSI.2013.2239115 [115] Cleary A, Glidle A, Laybourn PJR, Garcia-Blanco S, Pellegrini S et al. Integrating optics and microfluidics for time-correlated single-photon counting in lab-on-a-chip devices. Appl Phys Lett 91, 071123 (2007). doi: 10.1063/1.2772175 -
Access History
Article Metrics
-
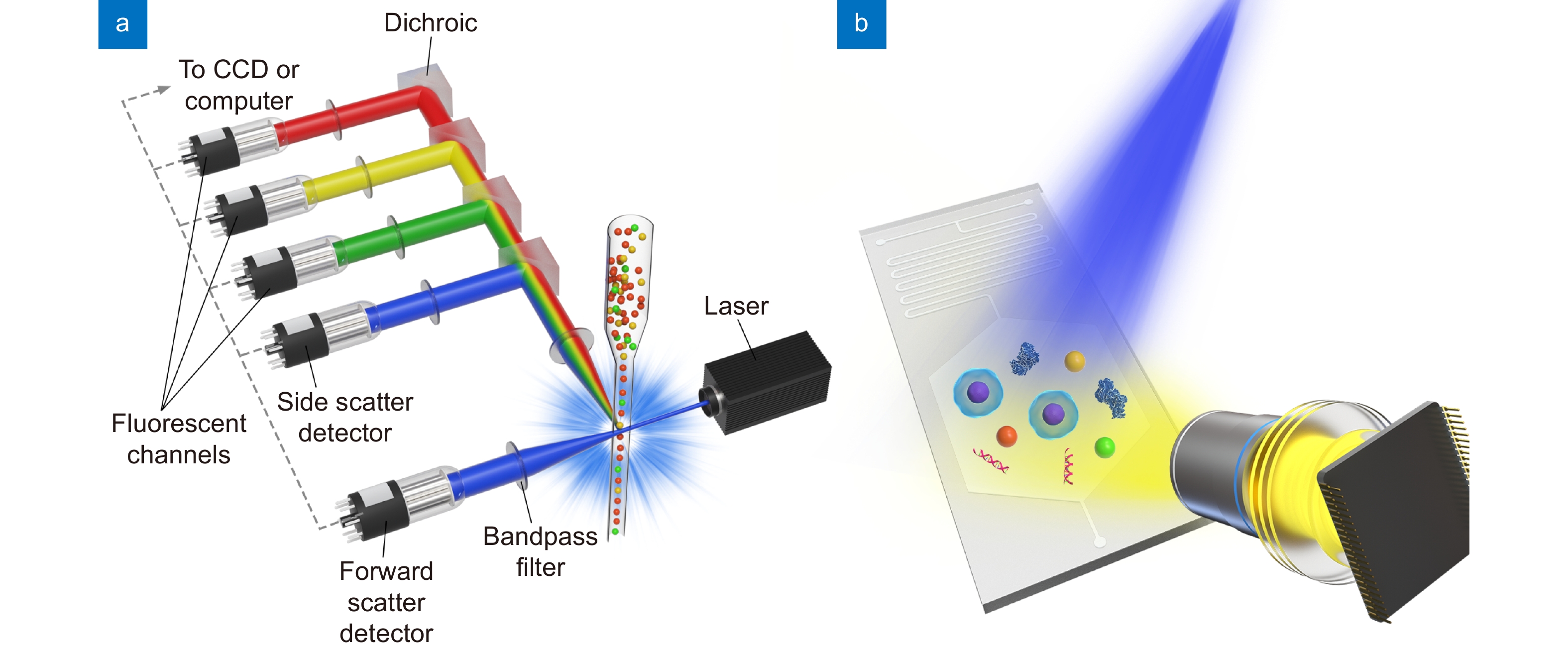
Figure 1.
Schematic of (a) the sheath fluid-assisted flow cytometer and (b) the Microfluidic Imaging Cytometer.
-
Figure 2.
Schematics and photographs for MIC devices with clinical application in (a) immunoassay, (b) semen analysis, (c) sickle cell disease screening. Figure reproduced with permission from: (a) ref.64, IEEE; (b) ref.67, The American Association for the Advancement of Science; (c) ref.71, under a Creative Commons Attribution 4.0 International License.
-
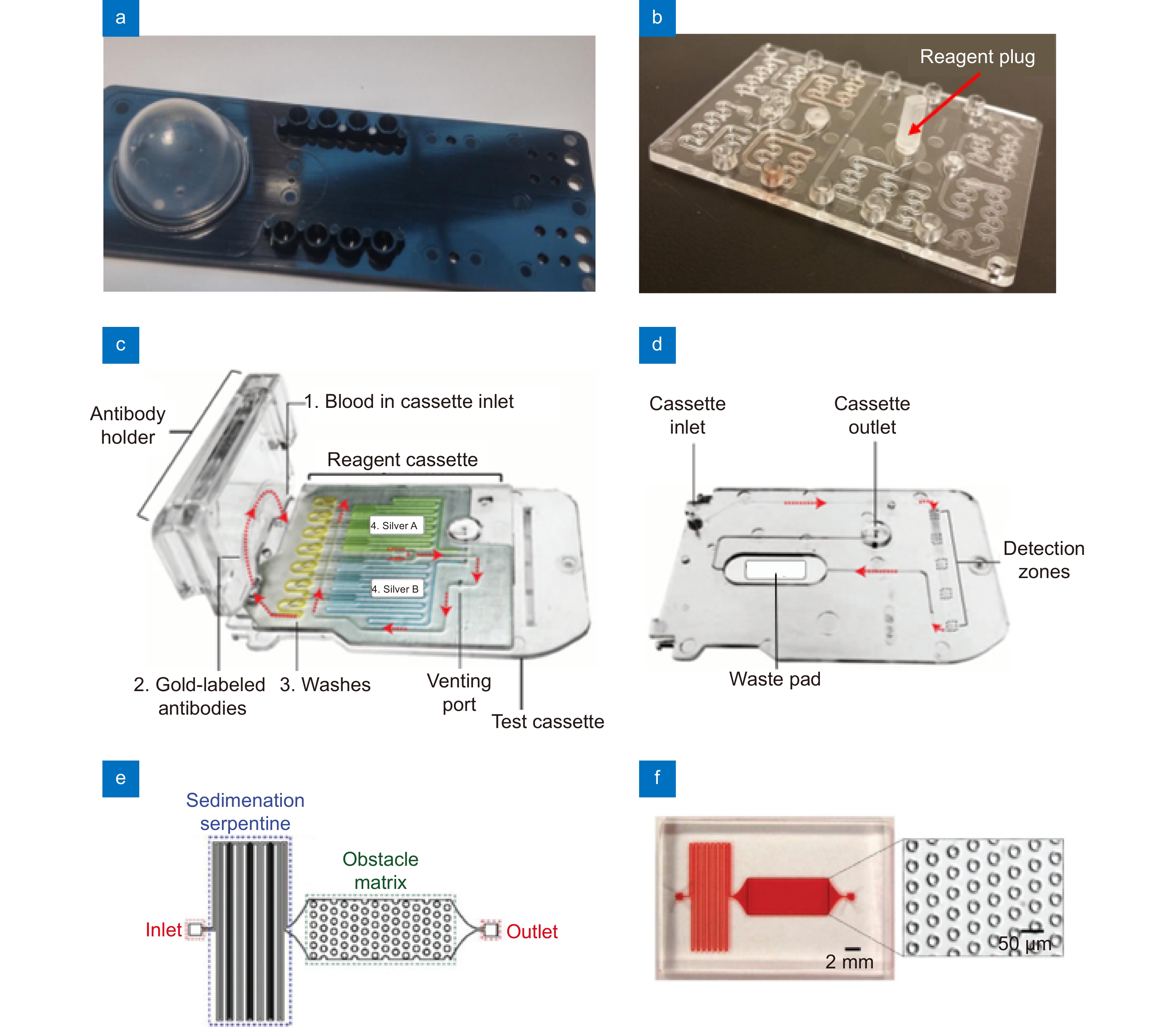
Figure 3.
(a) A photograph of an injection molded microfluidic chip with a bellows membrane to actuate the fluids. (b) A prototype microfluidic chip with serpentine resuspension regions and a reagent plug installed. (c) A photograph of a microfluidic reagent cassette containing pre-stored reagents. The microfluidic reagent cassette is part of a smartphone dongle for the testing of biomarkers such as HIV and syphilis antibodies. (d) The sequence of flow through the test cassette. From the inlet, fluids move through each detection zone sequentially, then flowing into a waste pad. (e) A schematic and (f) a photograph of a microfluidic chip for measuring CD64 expression cells from whole blood. The microfluidic structures were fabricated with sedimentation serpentine lanes and cell capture chambers with vertical posts. Figure reproduced with permission from: (a, b) ref.61, Open Access; (c, d) ref.75, The American Association for the Advancement of Science; (e, f) ref.76, Royal Society of Chemistry.
-
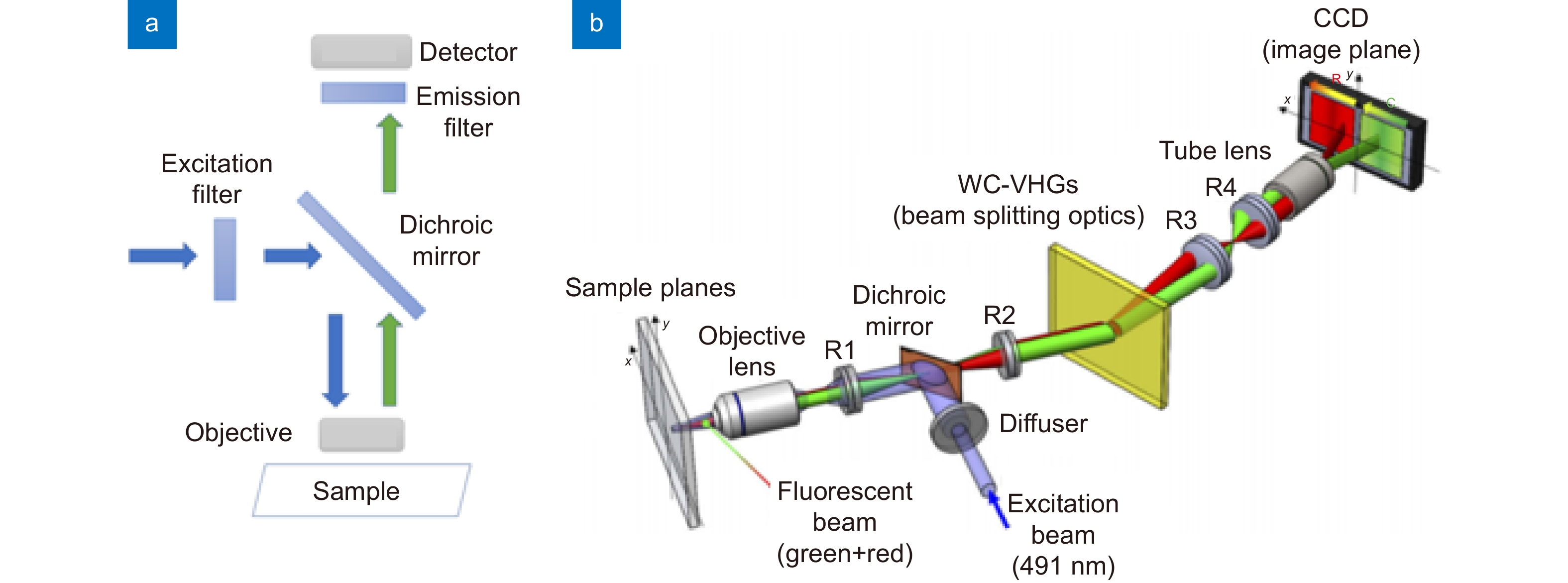
Figure 4.
(a) The schematic of an episcopic fluorescence microscope. (b) A volume holographic grating-based multi-color fluorescence microscope. Figure reproduced with permission from (b) ref.89, © The Optical Society.
-
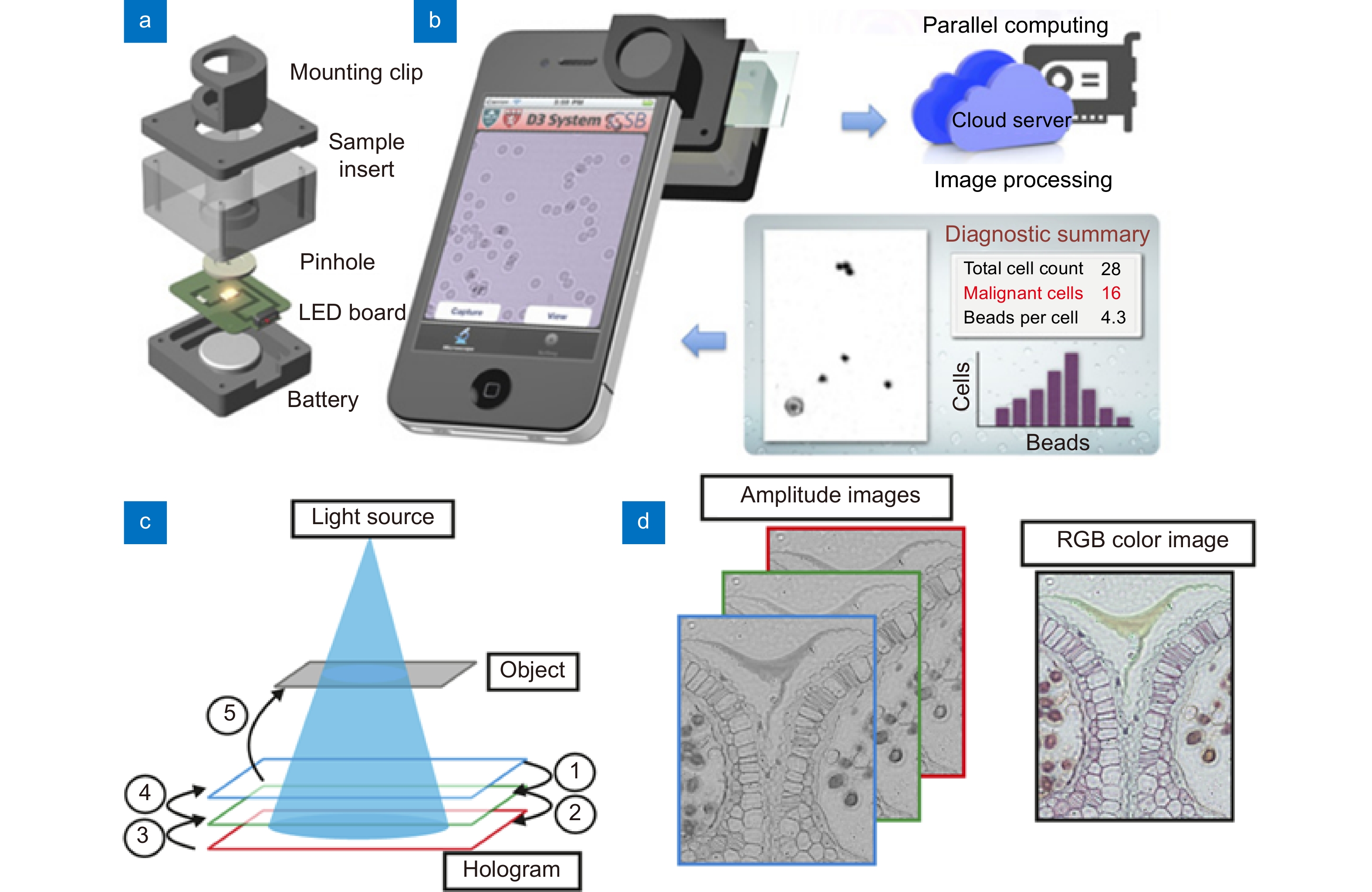
Figure 5.
(a) Snap-on module for a smartphone consisting of an LED powered by a coin battery, a pinhole for uniform illumination with partial coherence, and a sample mount. (b) The smartphone’s camera is used to record diffraction images of the specimen. The recorded images are transferred to a server via the cloud service for real-time image reconstruction and analyses, which can be returned to the smartphone in less than 2 min. (c) Lens free-imaging approach using illumination at primary RGB wavelengths simultaneously. Relative depths of the hologram planes used for phase retrieval. (d) Multi-wavelength phase retrieved amplitude images are placed into the RGB channels of a color reconstructed image. Figure reproduced with permission from: (a, b) ref.107, PNAS; (c, d) ref.108 © The Optical Society.
-
Figure 6.
(a) Illustration of TDI capture mode. The CCD’s delay time is matched with the cell’s velocity in the flow cell as it traverses the length of the camera’s field of view, providing an integrated readout of the cell’s fluorescent emission. Different spectral components originating from multiple fluorescent markers are separated horizontally along with the columns of the CCD. (b) Cell’s fluorescence spectrally resolved via the spectrograph. Figure reproduced with permission from ref.111, Copyright 2019 American Chemical Society.
-
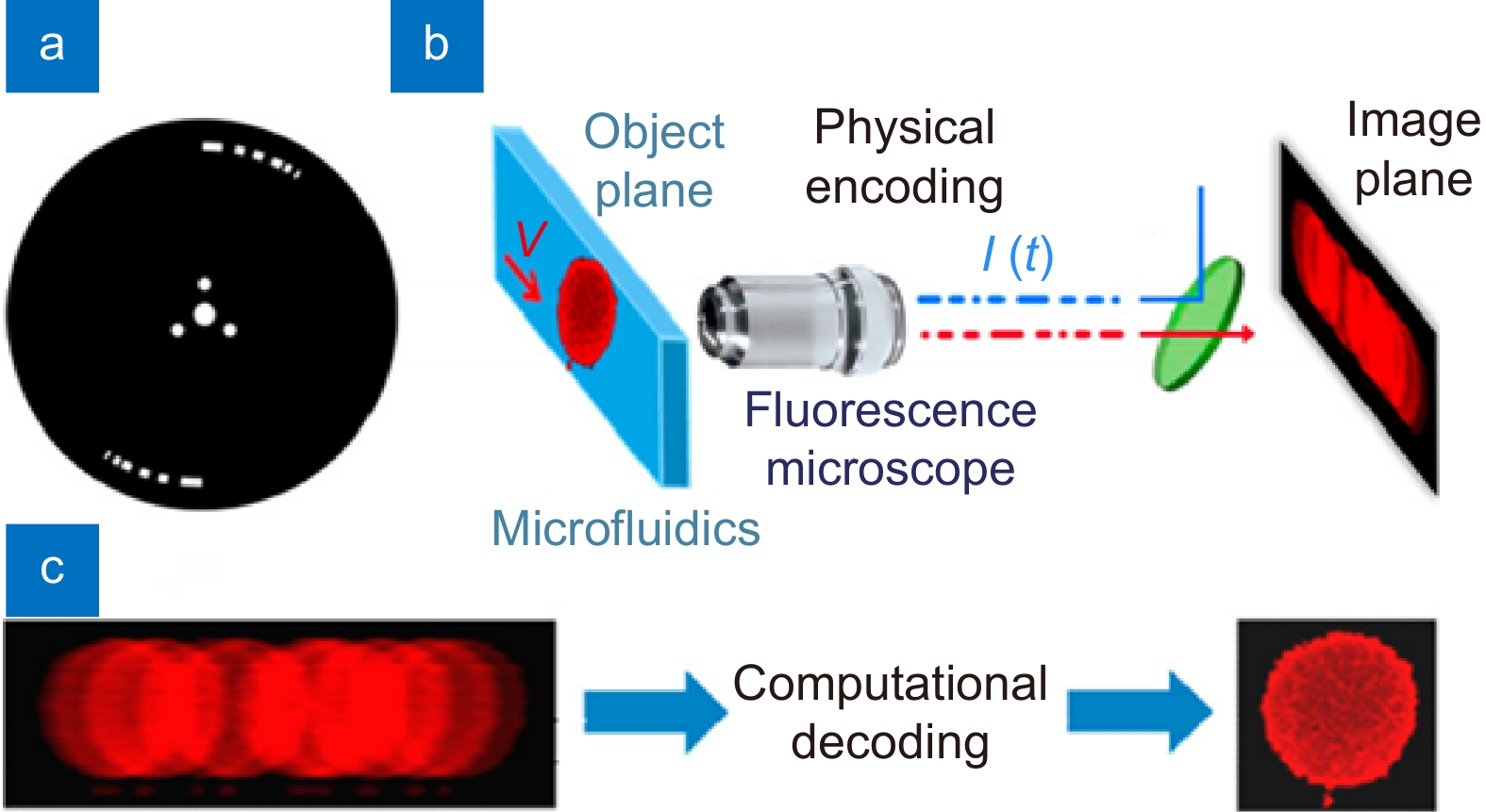
Figure 7.
Temporally coded excitation fluorescence microscope. (a) Chopper wheel that modulates the fluorescence excitation with a pseudo-random code. (b) Cells travel through a microfluidic device and the fluorescence emission is imaged by a microscope (0.65 NA, 40×) and recorded onto a camera. (c) Blur encoded images are captured by the camera and computationally decoded to produce near-diffraction-limited images. Figure reproduced with permission from ref.113, under Optica Open Access Publishing Agreement.

 E-mail Alert
E-mail Alert RSS
RSS

 DownLoad:
DownLoad: