| Citation: | Su DE, Li XY, Gao WD, Wei QH, Li HY et al. Smart palm-size optofluidic hematology analyzer for automated imaging-based leukocyte concentration detection. Opto-Electron Sci 2, 230018 (2023). doi: 10.29026/oes.2023.230018 |
Smart palm-size optofluidic hematology analyzer for automated imaging-based leukocyte concentration detection
-
Abstract
A critical function of flow cytometry is to count the concentration of blood cells, which helps in the diagnosis of certain diseases. However, the bulky nature of commercial flow cytometers makes such tests only available in hospitals or laboratories, hindering the spread of point-of-care testing (POCT), especially in underdeveloped areas. Here, we propose a smart Palm-size Optofluidic Hematology Analyzer based on a miniature fluorescence microscope and a microfluidic platform to lighten the device to improve its portability. This gadget has a dimension of 35 × 30 × 80 mm and a mass of 39 g, less than 5% of the weight of commercially available flow cytometers. Additionally, automatic leukocyte concentration detection has been realized through the integration of image processing and leukocyte counting algorithms. We compared the leukocyte concentration measurement between our approach and a hemocytometer using the Passing-Bablok analysis and achieved a correlation coefficient of 0.979. Through Bland-Altman analysis, we obtained the relationship between their differences and mean measurement values and established 95% limits of agreement, ranging from −0.93×103 to 0.94×103 cells/μL. We anticipate that this device can be used widely for monitoring and treating diseases such as HIV and tumors beyond hospitals. -
-
References
[1] Verso ML. The evolution of blood-counting techniques. Med Hist 8, 149–158 (1964). doi: 10.1017/S0025727300029392 [2] Yan X, Song JF, Zhang L, Li X. Analysis of risk factors for multidrug-resistant organisms in diabetic foot infection. BMC Endocr Disord 22, 46 (2022). doi: 10.1186/s12902-022-00957-0 [3] Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM et al. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 117, 556–562 (2004). doi: 10.1016/j.amjmed.2004.06.022 [4] Saleem A, Mubeen A, Akhtar MF, Zeb A. Polystichum braunii ameliorates airway inflammation by attenuation of inflammatory and oxidative stress biomarkers, and pulmonary edema by elevation of aquaporins in ovalbumin-induced allergic asthmatic mice. Inflammopharmacology 30, 639–653 (2022). doi: 10.1007/s10787-022-00944-w [5] Chmielewski PP, Strzelec B. Elevated leukocyte count as a harbinger of systemic inflammation, disease progression, and poor prognosis: a review. Folia Morphol 77, 171–178 (2018). doi: 10.5603/FM.a2017.0101 [6] Athale UH, Kaste SC, Bodner SM, Ribeiro RC. Splenic rupture in children with hematologic malignancies. Cancer 88, 480–490 (2000). doi: 10.1002/(SICI)1097-0142(20000115)88:2<480::AID-CNCR31>3.0.CO;2-I [7] Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit Care Med 31, 104–112 (2003). doi: 10.1097/00003246-200301000-00017 [8] Cheng XH, Irimia D, Dixon M, Sekine K, Demirci U et al. A microfluidic device for practical label-free CD4+ T cell counting of HIV-infected subjects. Lab Chip 7, 170–178 (2007). doi: 10.1039/B612966H [9] Vembadi A, Menachery A, Qasaimeh MA. Cell cytometry: review and perspective on biotechnological advances. Front Bioeng Biotechnol 7, 147 (2019). doi: 10.3389/fbioe.2019.00147 [10] Sami MA, Tayyab M, Parikh P, Govindaraju H, Hassan U. A modular microscopic smartphone attachment for imaging and quantification of multiple fluorescent probes using machine learning. Analyst 146, 2531–2541 (2021). doi: 10.1039/D0AN02451A [11] Rabha D, Biswas S, Hatiboruah D, Das P, Rather MA et al. An affordable, handheld multimodal microscopic system with onboard cell morphology and counting features on a mobile device. Analyst 147, 2859–2869 (2022). doi: 10.1039/D1AN02317A [12] Avci MB, Yasar SD, Cetin AE. An optofluidic platform for cell-counting applications. Anal Methods 15, 2244–2252 (2023). doi: 10.1039/D3AY00344B [13] Gorti V, Kaza N, Williams EK, Lam WA, Robles FE. Compact and low-cost deep-ultraviolet microscope system for label-free molecular imaging and point-of-care hematological analysis. Biomed Opt Express 14, 1245–1255 (2023). doi: 10.1364/BOE.482294 [14] Lei C, Kobayashi H, Wu Y, Li M, Isozaki A et al. High-throughput imaging flow cytometry by optofluidic time-stretch microscopy. Nat Protoc 13, 1603–1631 (2018). doi: 10.1038/s41596-018-0008-7 [15] Gӧrӧcs Z, Tamamitsu M, Bianco V, Wolf P, Roy S et al. A deep learning-enabled portable imaging flow cytometer for cost-effective, high-throughput, and label-free analysis of natural water samples. Light Sci Appl 7, 66 (2018). doi: 10.1038/s41377-018-0067-0 [16] Holzner G, Du Y, Cao XB, Choo J, deMello AJ et al. An optofluidic system with integrated microlens arrays for parallel imaging flow cytometry. Lab Chip 18, 3631–3637 (2018). doi: 10.1039/C8LC00593A [17] Gӧrӧcs Z, Tamamitsu M, Bianco V, Wolf P, Roy S et al. Portable imaging flow cytometer using deep learning based holographic image reconstruction. In Conference on Lasers and Electro-Optics SM4H. 2 (OSA, 2019);http://doi.org/10.1364/CLEO_SI.2019.SM4H.2. [18] Kim S, Streets AM, Lin RR, Quake SR, Weiss S et al. High-throughput single-molecule optofluidic analysis. Nat Methods 8, 242–245 (2011). doi: 10.1038/nmeth.1569 [19] Nitta N, Sugimura T, Isozaki A, Mikami H, Hiraki K et al. Intelligent image-activated cell sorting. Cell 175, 266–276.e13 (2018). doi: 10.1016/j.cell.2018.08.028 [20] Zhang H, Lu MY, Xiong Z, Yang J, Tan MY et al. Rapid trapping and tagging of microparticles in controlled flow by in situ digital projection lithography. Lab Chip 22, 1951–1961 (2022). doi: 10.1039/D2LC00186A [21] Wang ZQ, Zhu LX, Zhang H, Li G, Yi CQ et al. Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning. Nat Methods 18, 551–556 (2021). doi: 10.1038/s41592-021-01058-x [22] Gonzalez G, Chiappone A, Dietliker K, Pirri CF, Roppolo I. Fabrication and functionalization of 3D printed polydimethylsiloxane‐based microfluidic devices obtained through digital light processing. Adv Mater Technol 5, 2000374 (2020). doi: 10.1002/admt.202000374 [23] Prakash M, Gershenfeld N. Microfluidic bubble logic. Science 315, 832–835 (2007). doi: 10.1126/science.1136907 [24] Saggiomo V, Velders AH. Simple 3D printed scaffold-removal method for the fabrication of intricate microfluidic devices. Adv Sci 2, 1500125 (2015). doi: 10.1002/advs.201500125 [25] Lee Y, Kim B, Choi S. Integrated microflow cytometry for portable immunophenotypic cell analysis. Sens Actuators A Phys 309, 112038 (2020). doi: 10.1016/j.sna.2020.112038 [26] Maleki T, Fricke T, Quesenberry JT, Todd PW, Leary JF. Point-of-care, portable microfluidic blood analyzer system. Proc SPIE 8251, 82510C (2012). [27] Guo CL, Blair GJ, Sehgal M, Jimka FNS, Bellafard A et al. Miniscope-LFOV: a large-field-of-view, single-cell-resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals. Sci Adv 9, eadg3918 (2023). doi: 10.1126/sciadv.adg3918 [28] Zong WJ, Wu RL, Chen SY, Wu JJ, Wang HB et al. Miniature two-photon microscopy for enlarged field-of-view, multi-plane and long-term brain imaging. Nat Methods 18, 46–49 (2021). doi: 10.1038/s41592-020-01024-z [29] De Groot A, Van Den Boom BJ, Van Genderen RM, Coppens J, Van Veldhuijzen J et al. NINscope, a versatile miniscope for multi-region circuit investigations. eLife 9, e49987 (2020). doi: 10.7554/eLife.49987 [30] Zong WJ, Wu RL, Li ML, Hu YH, Li YJ et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Methods 14, 713–719 (2017). doi: 10.1038/nmeth.4305 [31] Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y et al. Miniaturized integration of a fluorescence microscope. Nat Methods 8, 871–878 (2011). doi: 10.1038/nmeth.1694 [32] Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology E-Book (Elsevier, Amsterdam, 2017). [33] Bilić-Zulle L. Comparison of methods: passing and Bablok regression. Biochem Med 21, 49–52 (2011). [34] Smith S, Madzivhandila P, Sewart R, Govender U, Becker H et al. Microfluidic cartridges for automated, point-of-care blood cell counting. SLAS Technol 22, 176–185 (2017). doi: 10.1177/2211068216677820 [35] Osei-Bimpong A, Jury C, McLean R, Lewis SM. Point-of-care method for total white cell count: an evaluation of the HemoCue WBC device. Int J Lab Hematol 31, 657–664 (2009). doi: 10.1111/j.1751-553X.2008.01093.x [36] Al-Riyami AZ, Al-Khabori M, Al-Hadhrami RM, Al-Azwani IS, Davis HM et al. The pneumatic tube system does not affect complete blood count results; a validation study at a tertiary care hospital. Int J Lab Hematol 36, 514–520 (2014). doi: 10.1111/ijlh.12180 -
Supplementary Information
Supplementary_Video_1 
Supplementary information for Smart Palm-size Optofluidic Hematology Analyzer for automated imaging-based leukocyte concentration detection 
-
Access History
Article Metrics
-
Figure 1.
Principle and construction of the Palm-size Optofluidic Hematology Analyzer. (a) The photograph and model diagrams of the Palm-size Optofluidic Hematology Analyzer. (b) The model diagram of a miniature fluorescence microscope. (c) The optical path design of a miniature fluorescence microscope. (d) Results of the USAF target at various axial positions imaged by the miniature fluorescence microscope. Z stands for object distance. (e) The lateral resolution of miniature fluorescence microscope as a function of object distance. (f) The model diagram of the designed microfluidic chip with top and front views of the profiles.
-
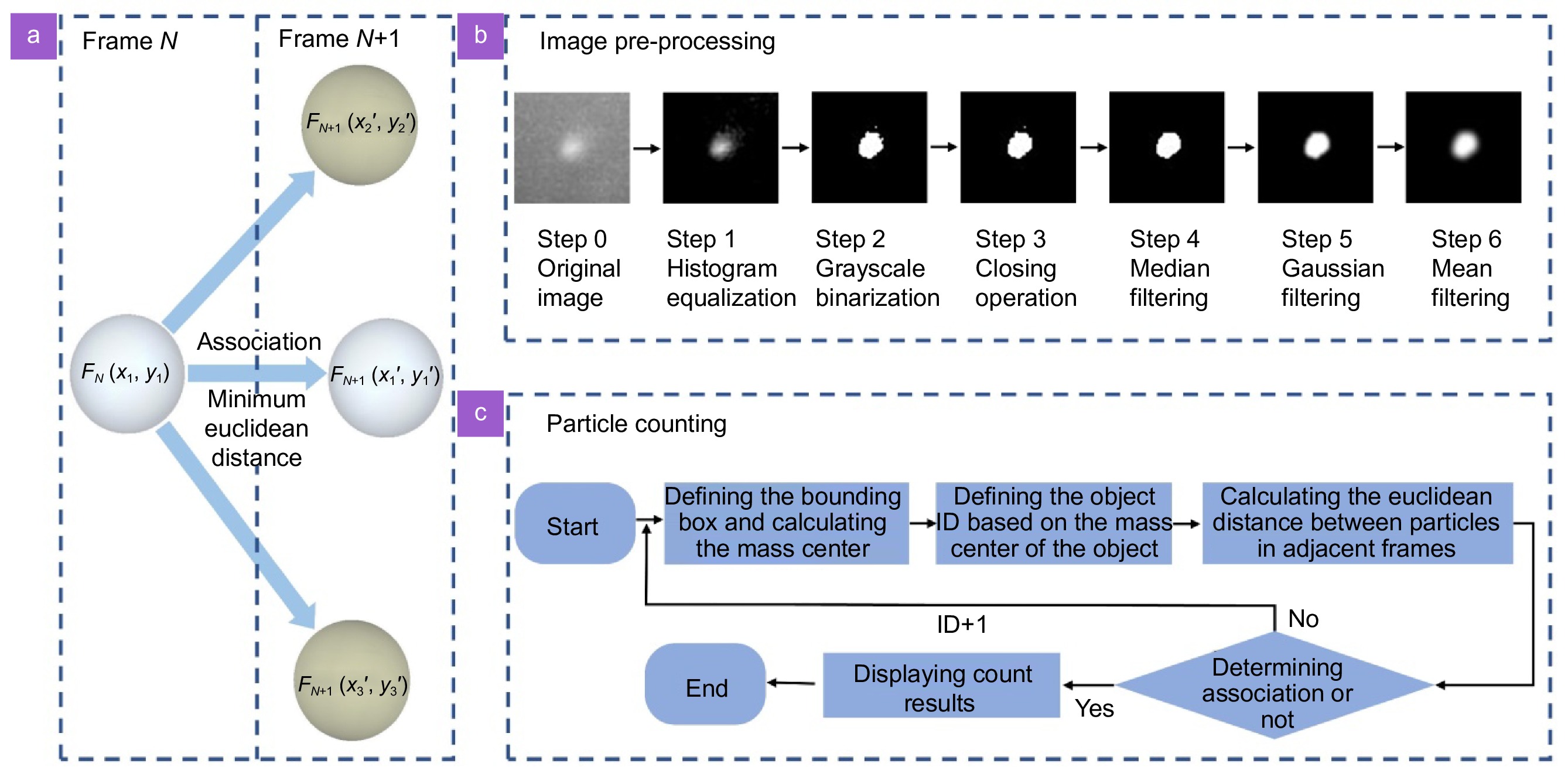
Figure 2.
(a) Particle centroid tracking principle. (b) Flow chart of image pre-processing. (c) Flow chart of particle counting.
-
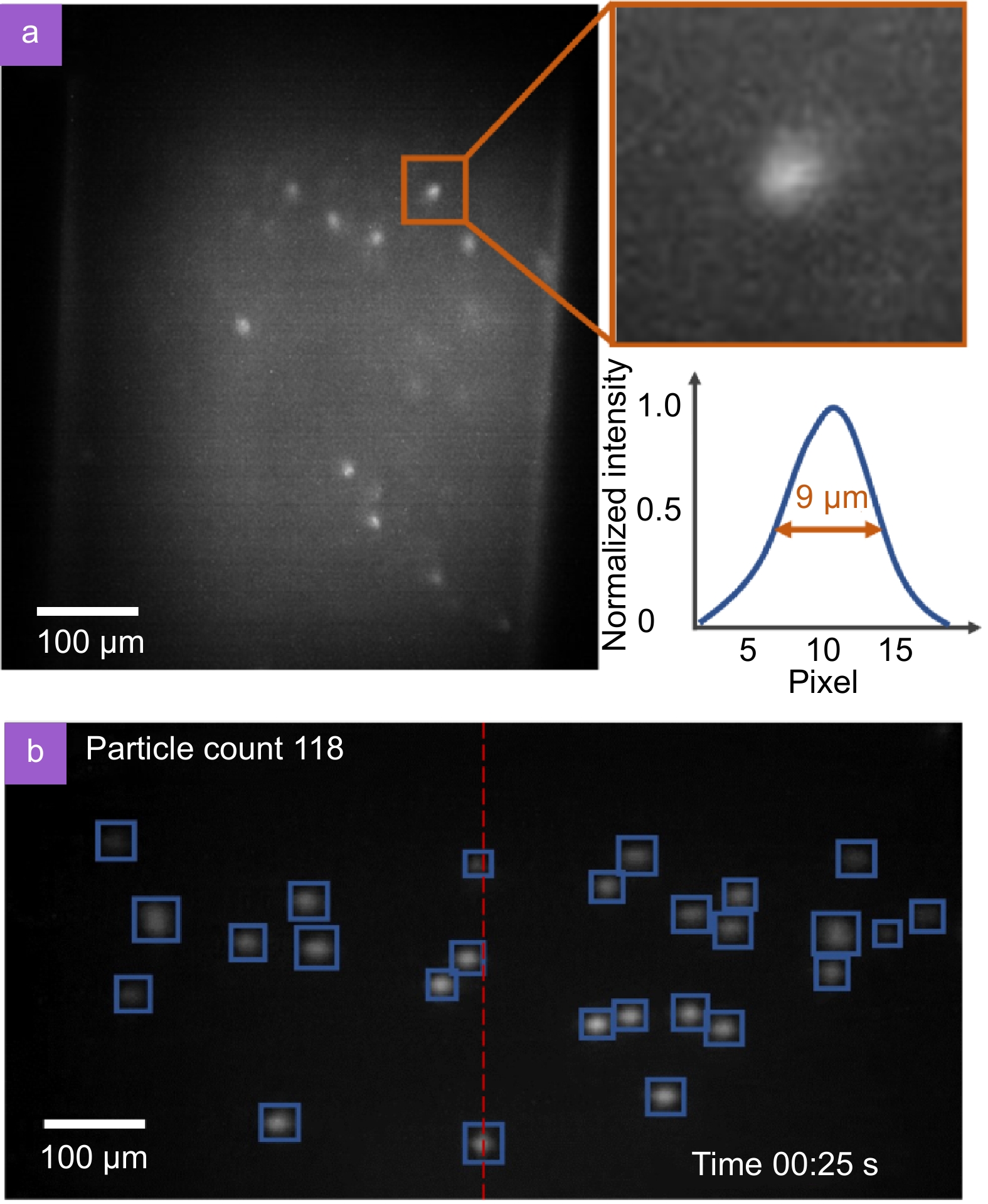
Figure 3.
(a) Leukocytes in a channel captured by a miniature fluorescence microscope, with a magnified view of one of the cells and its profile curve. (b) Working process of the particle counting algorithm.
-
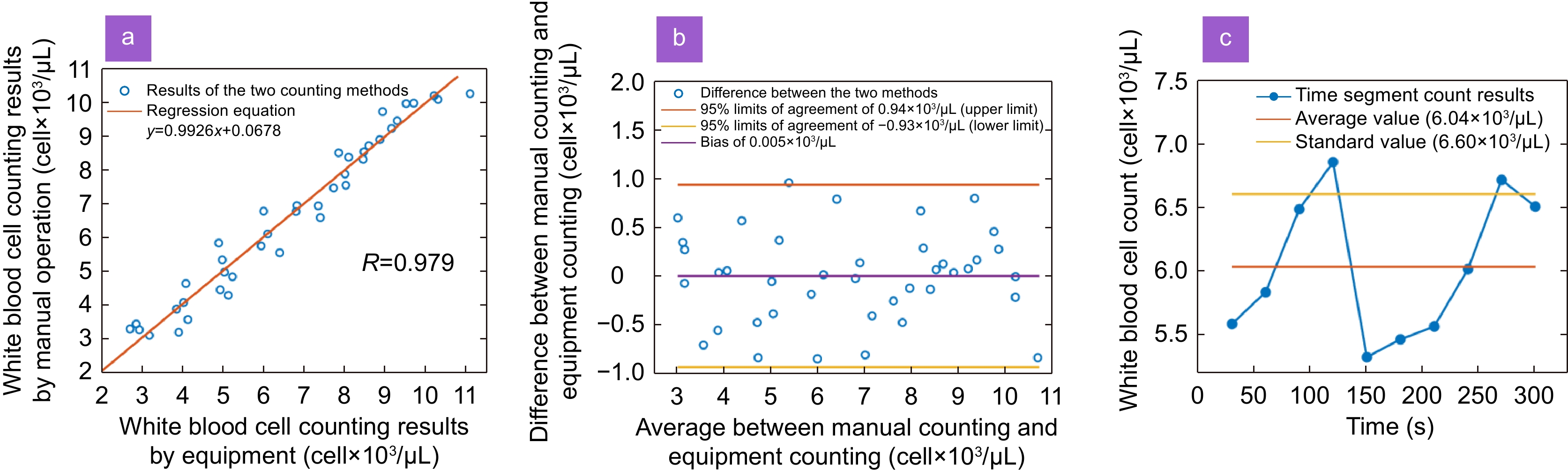
Figure 4.
(a) Scatter diagram and regression equation of total white blood cells from both methods analyzed by Passing-Bablok regression analysis, sample number = 40. Regression equation: y = 0.9926 x + 0.0678, correlation coefficient R = 0.979; 95% confidence interval for slope 0.7955 to 1.0304 and for intercept –0.4380 to 0.9189. (b) The Bland-Altman analysis between the average and difference of the total white blood cells calculated by the two methods. The orange and yellow lines represent the upper and lower LOA, respectively, and the purple line represents the bias of the average count difference from 0. (c) The white blood cell counting results obtained from a patient's whole blood using our Palm-size Optofluidic Hematology Analyzer. The orange line represents the average of 10-count results, and the yellow line represents the standard value obtained by the hemocytometer.

 E-mail Alert
E-mail Alert RSS
RSS


 DownLoad:
DownLoad: